Survey Results: EU MDR Readiness Check 40 Days before the Deadline

DATE
April 15, 2021
AUTHOR
Alex | Product Manager
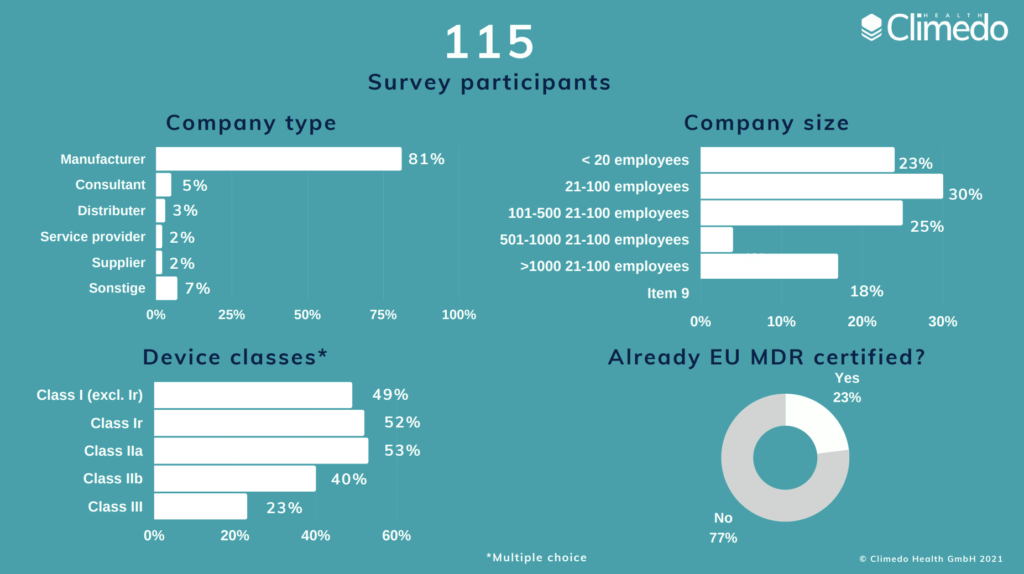
There are just a few weeks left until the new validity date of the EU MDR on the 26th of May. Following our first survey back in 2020, which was published shortly before the postponement of the EU MDR deadline, we wanted to find out where medical device manufacturers stand now with regards to the implementation. We therefore created a second survey to explore whether medical device manufacturers are more prepared for the new regulations, which challenges they continue to face and whether the additional 12 months helped with the implementation. The survey ran between March and April and we collected responses from 115 participants all over Europe, 81% of which were medical device manufacturers. All risk classes were represented in the survey. 23% were already MDR-certified.
EU MDR compliance is still a major challenge
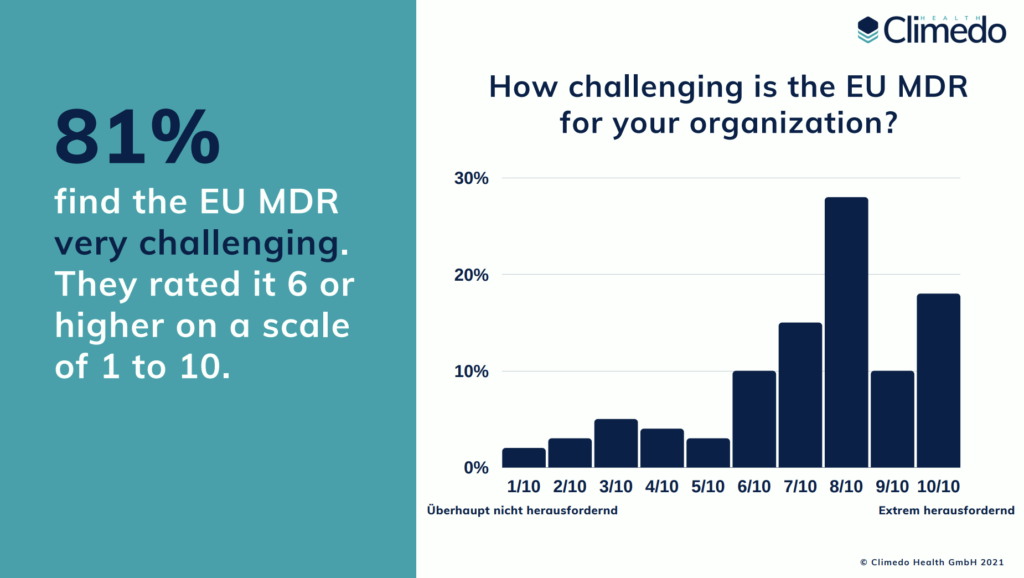
Our survey indicates that the implementation of the EU MDR still poses a major challenge for most manufacturers. 81% of respondents consider the MDR to be “very challenging”, compared to 77% in 2020. The top three challenges they encounter are increased resources or costs (70%), lack of clarity (59%) and the required clinical investigations (54%). We also asked our participants how much additional cost they expect their company to incur due to the new regulations. A third (31%) of respondents estimated that the MDR will add costs of between 5 and 10% of the annual revenue, 13% even believed that it could be more than 10%. According to the results, most manufacturers (64%) also invest more than 5 additional hours per week in meeting the EU MDR demands.
Postponement of the deadline and status of Notified Bodies
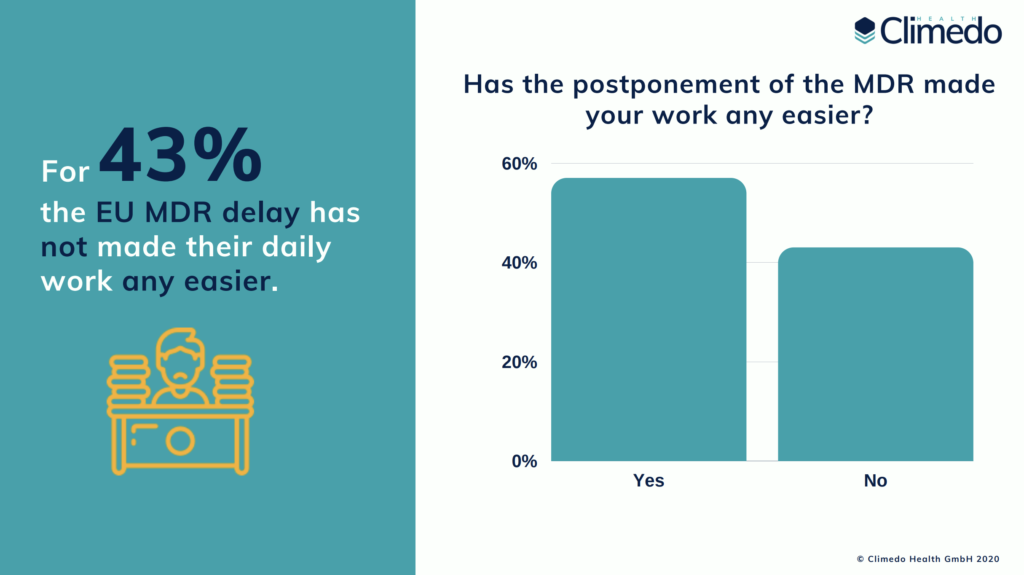
In April 2020, the EU MDR validity date was postponed by one year due to the current pandemic. Although several participants claim that this helped with planning and understanding the requirements, 43% still responded that the delay has not made their daily work any easier. They argue that the postponement only led to a shift in priorities and the ambiguity relating to the regulations still remains. However, the number of manufacturers who have a MDR-certified notified body increased from 52% in 2020 to 72%. For nearly 40% of these participants, finding a Notified Body was “not at all challenging”. With regards to virtual audits, 61% claimed they have made use of them already or plan to do so in the future.
Clinical Data Capture
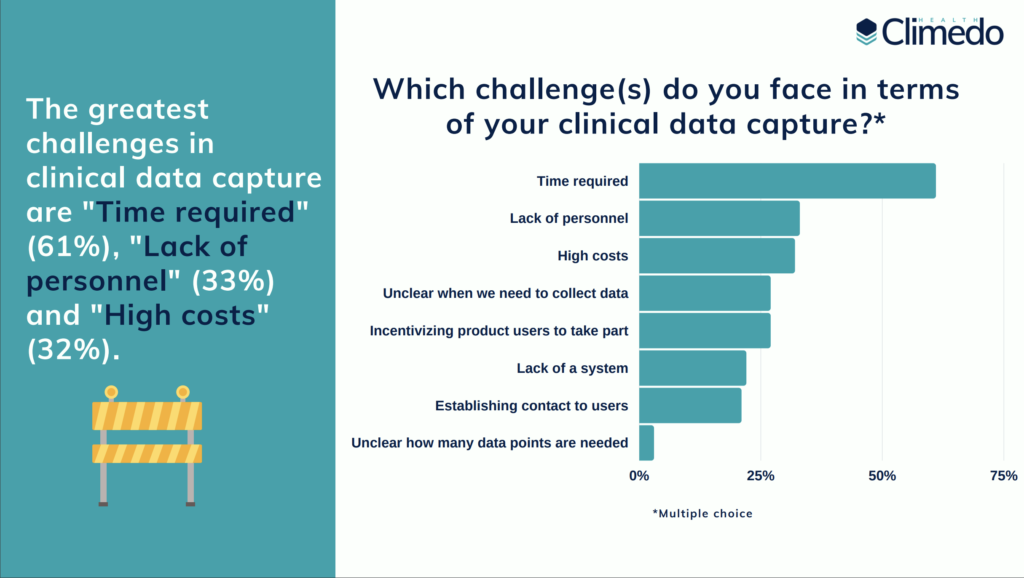
When it comes to clinical data capture 46% still rely on paper, 61% use Excel sheets. Interestingly, more than half of all survey participants see “no benefits at all” in these solutions. Merely 25% of participants rely on an Electronic Data Capture system for their clinical data collection, although 59% believe EDC-Software to be “practical in use” and 50% find it to be “time saving”. When we asked the participants about their main challenges in clinical data capture, the three most popular answers included “time required” (61%), “lack of personnel” (33%), and “high costs” (32%).
A look at the future
While the EU MDR certainly poses many challenges and difficulties for medical device manufacturers, there are also some advantages. For instance, “Traceability” and “Transparency” were cited by 43 and 40% respectively as the greatest perceived benefits. However, 32% of respondents saw “no benefits at all”. We also asked the participants what they would like to see from the EU commission. The results revealed that 75% would like to see “clearer guidelines”, 50% would like to see “more technical support” and 39% would like to see “training or informative events”.
Still need help getting MDR compliant?
As the results show, the EU MDR continues to be very challenging for most medical device companies. If you need support in digitalizing your clinical data processes to help you save valuable time, feel free to book a quick software demo with us!
You can also read this article via Medium.