Climedo for medical device manufacturers
Clinical Trials for Medical Devices Made Easy
Climedo helps you to save time and costs, improve data quality, and easily conduct surveys for your medical device studies.
Climedo helps you to save time and costs, improve data quality, and easily conduct surveys for your medical device studies.


The EU-MDR presents manufacturers with numerous new challenges. Experts believe that in Europe, 10% of medical technology companies are threatened in their existence due to the MDR and that around 30% of medical devices must be withdrawn from the market or cannot be recertified in time.
With Climedo you ensure the performance and safety of your medical devices over their entire life cycle. Benefit from a user-friendly and secure solution for your medical device studies, save time and money and increase your data quality at the same time. With our survey tool, you can carry out simple surveys that your product users can conveniently answer on the device of their choice (smartphone, tablet, computer). All data is processed in compliance with the highest data protection requirements.

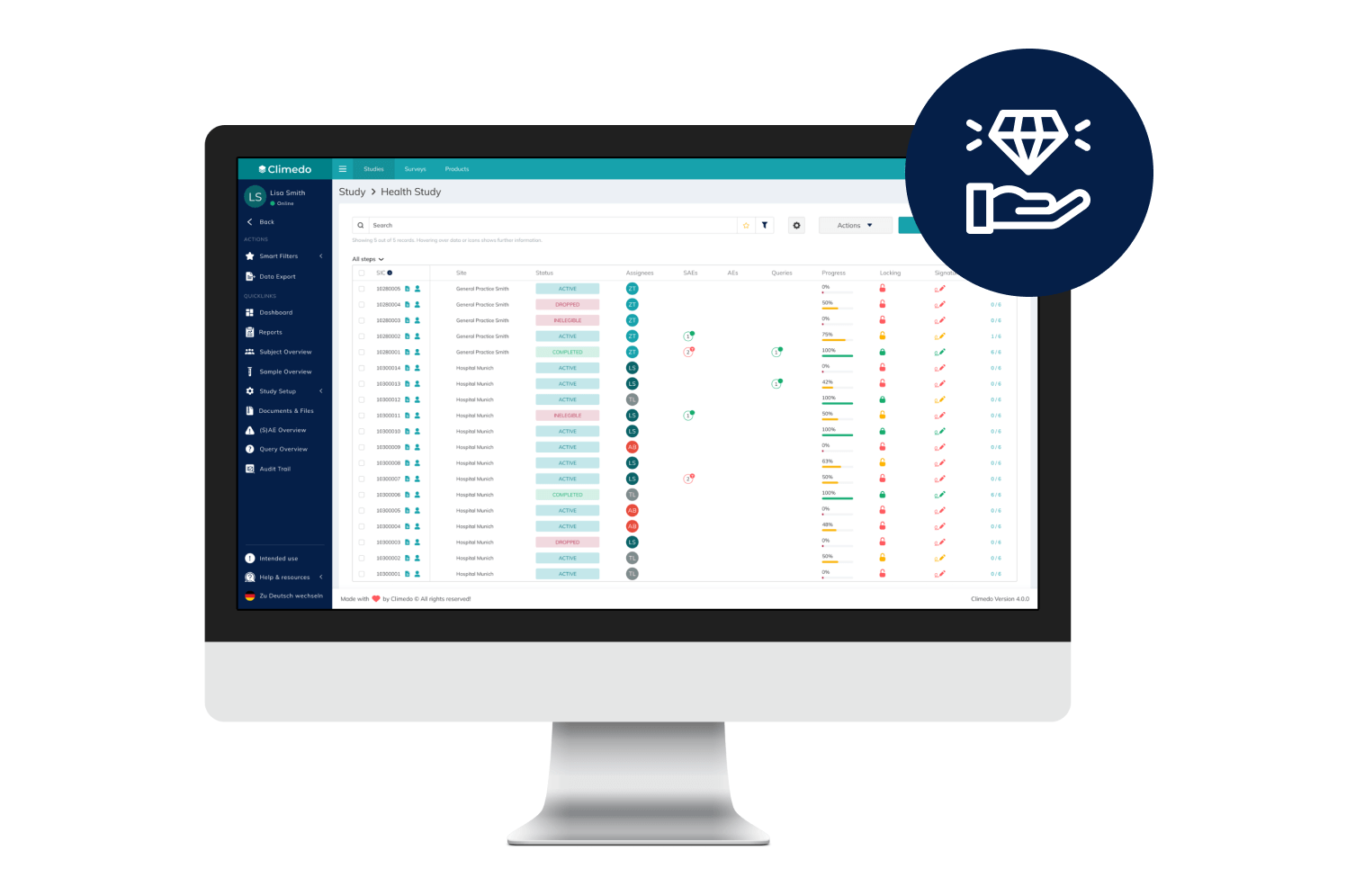
EDC
Cost-efficient, automated and scalable: Capture data for your clinical studies and surveys electronically with our EDC system – all in one place.
Our EDC System
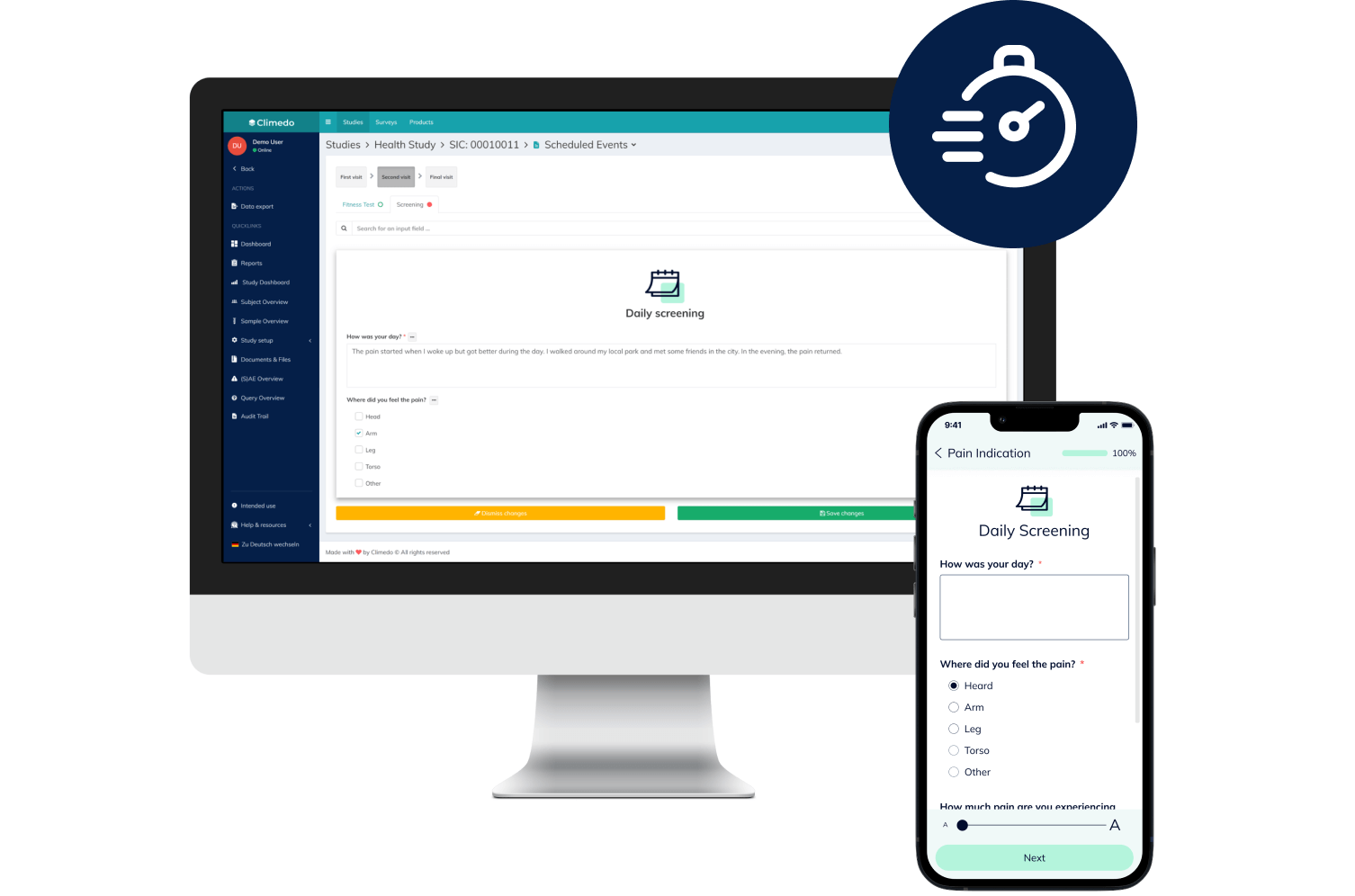
eCOA & ePRO
Convenient, secure and fast: Digitally capture all data reported by your patients in the course of a clinical study – on any device and with no app.
Our ePRO Solutions
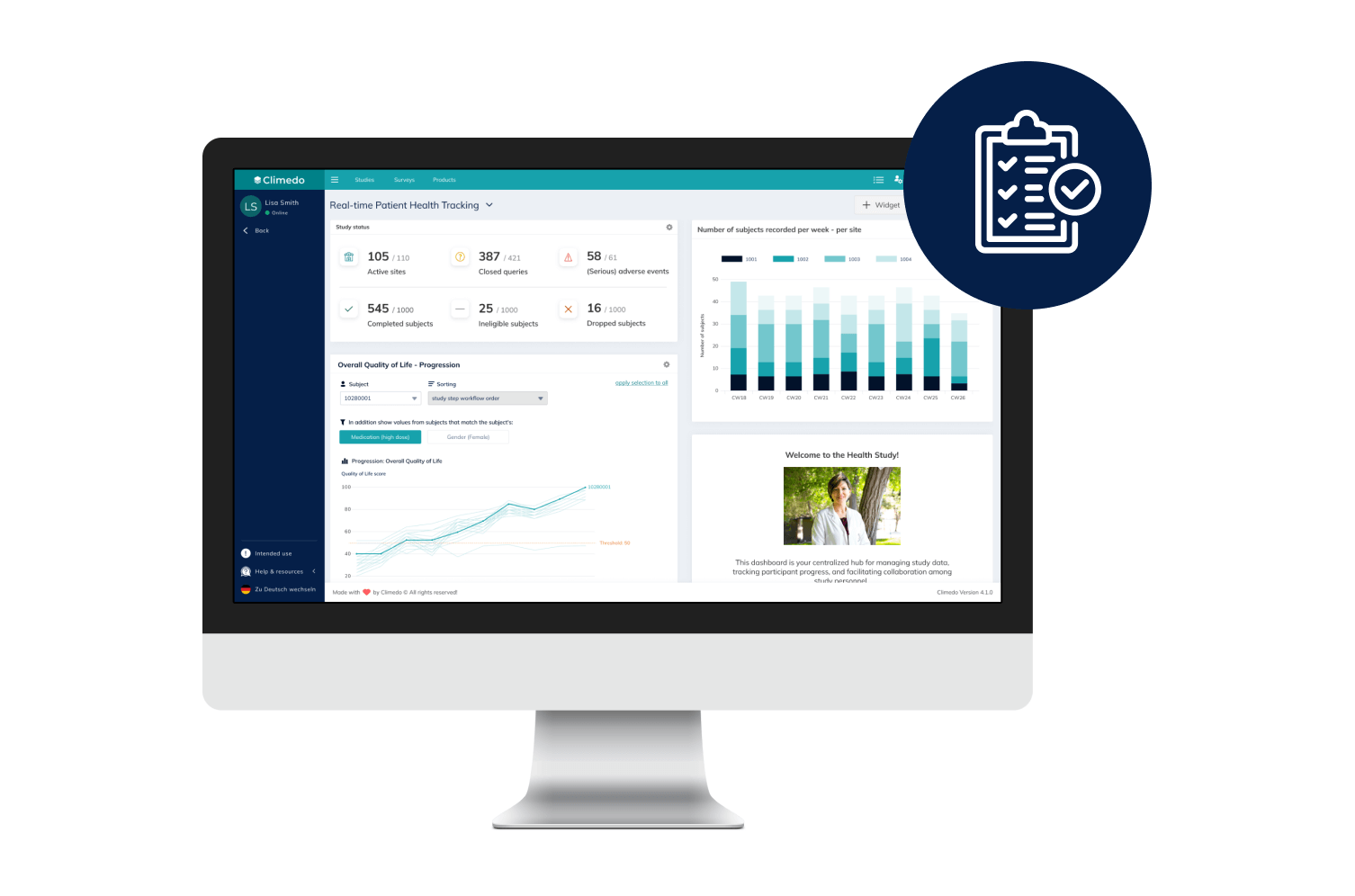
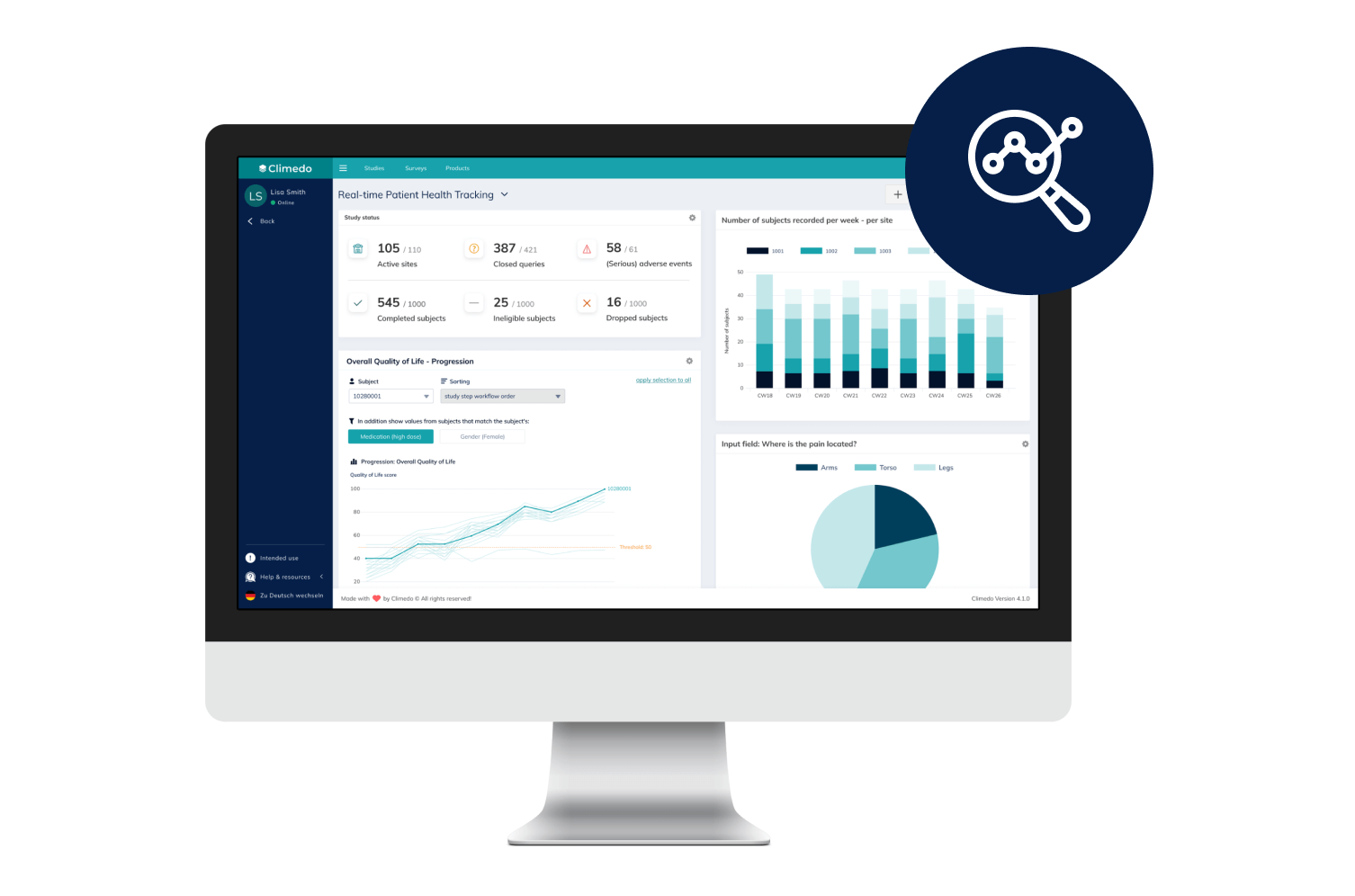
Real-Time Dashboards
Our interactive dashboards enable you to see real-time visualizations of your clinical trial data and compare patient cohorts across different sites.
Our Real-Time Dashboards
eConsent
Simplifies your administrative processes at the start of a study. Study participants are informed digitally.
Our eConsent
Hybrid Trials
Implement clinical trials in a patient-centered, flexible, and decentralized manner, during the pandemic and beyond.
Our Hybrid Trial Solutions

We'll show you how to start a clinical trial or survey and address your specific needs and desires.
Climedo's intuitive user interface means you'll be onboarded in just a few hours, without the need for programming skills. Get started with your project right away.
Patient centricity and decentralized study designs accelerate your recruitment and increase patient compliance by up to 90%.
Predefined workflows with complex decision trees including automatic plausibility and validation checks improve the data quality from the time of input.
Achieve significant time savings of up to 49% thanks to reusable form templates and the possibility of decentralized study designs and remote monitoring.
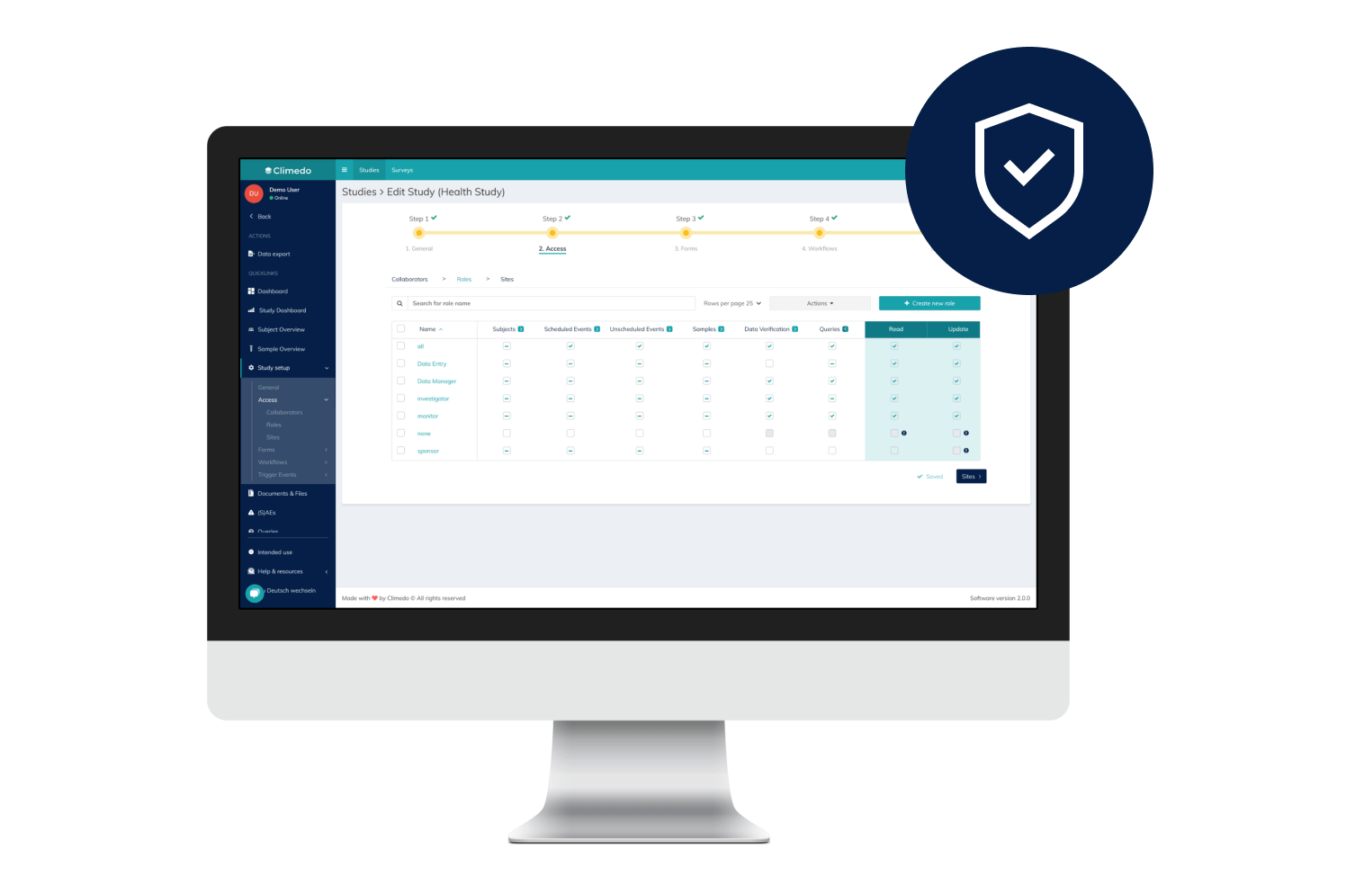
Our cloud-based solution has been tested by state authorities and offers a granular roles and rights assignment for different system users.
Dashboards and smart filter views allow you to view all results in real time and thus anticipate potential problems. Data exports are possible in common formats.


Prof. Dr. Christoph Schmitz
Chief Medical Officer, Auto Tissue Berlin

Christiane Weis, M.Sc
Clinical Department, Gelita Medical
Thanks to the simple handling (independent of location or device), the intuitive usage and the automated reminders, you'll see higher participation and completion rates.
Direct patient feedback and paperless data collection will improve your data quality. With Climedo, the error-prone transfer of paper to Excel sheets and illegible handwriting become a thing of the past.
Faster results thanks to automated processes, reusable forms and decentralized study designs as well as remote monitoring.
