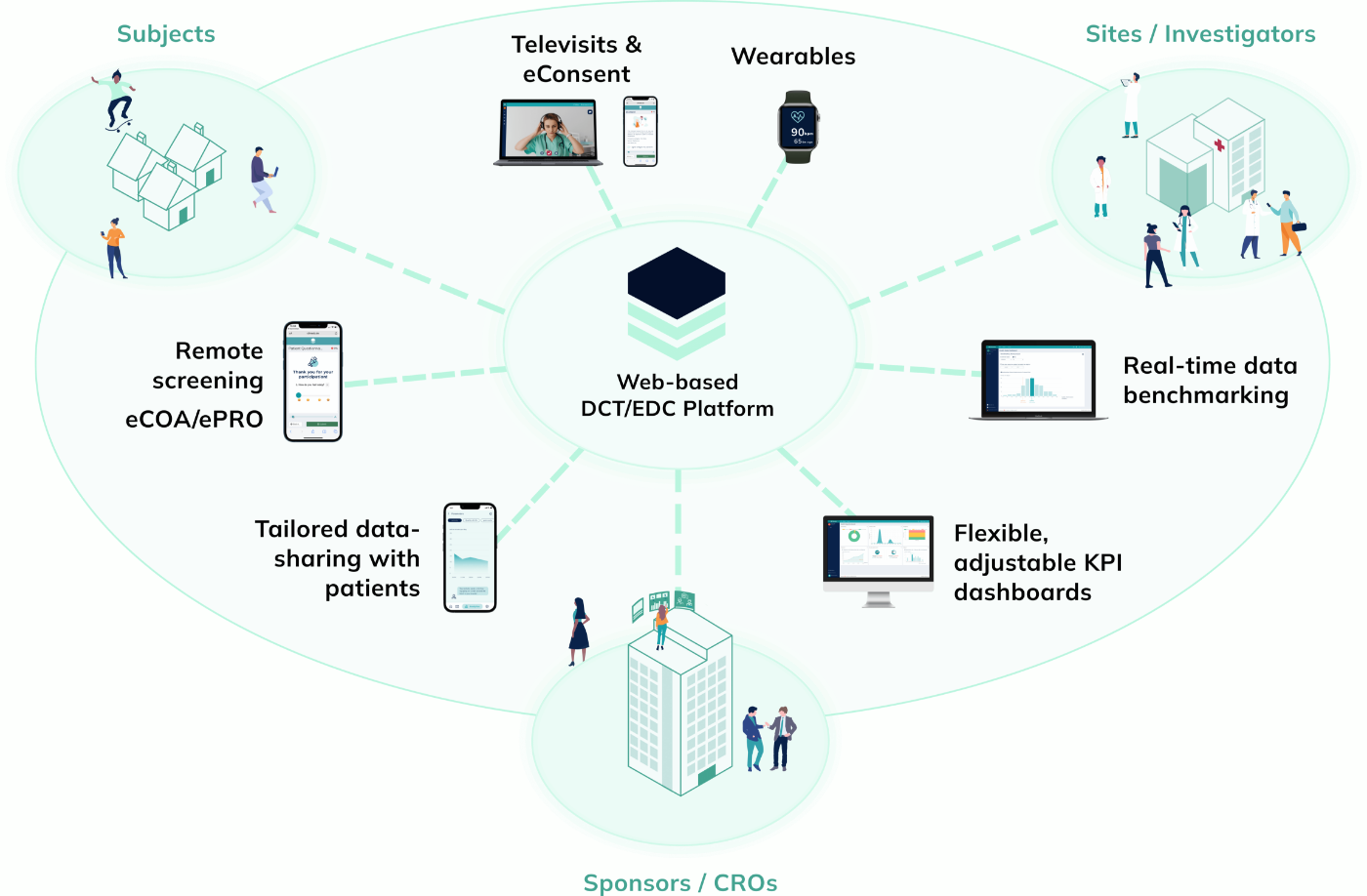
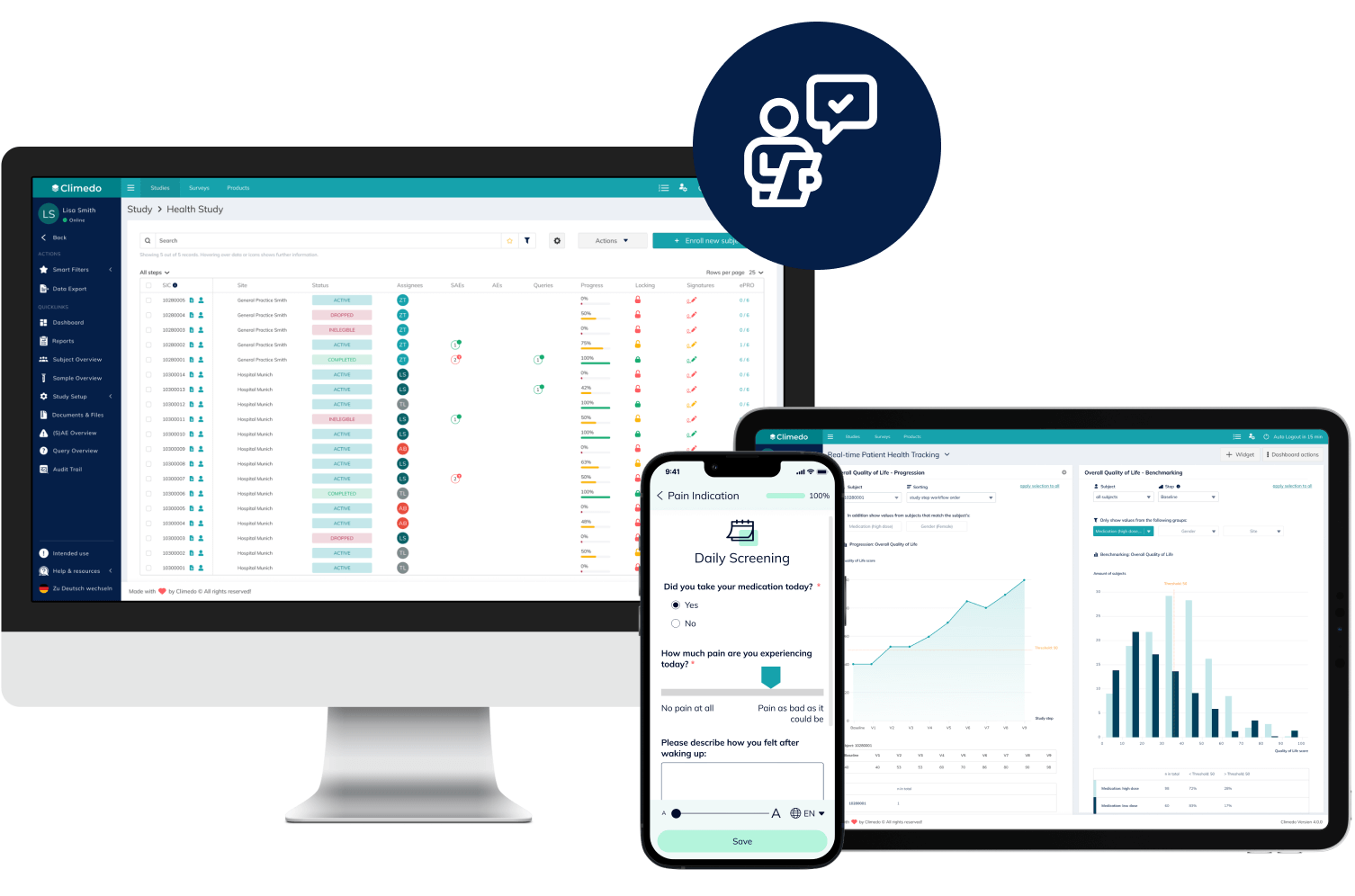
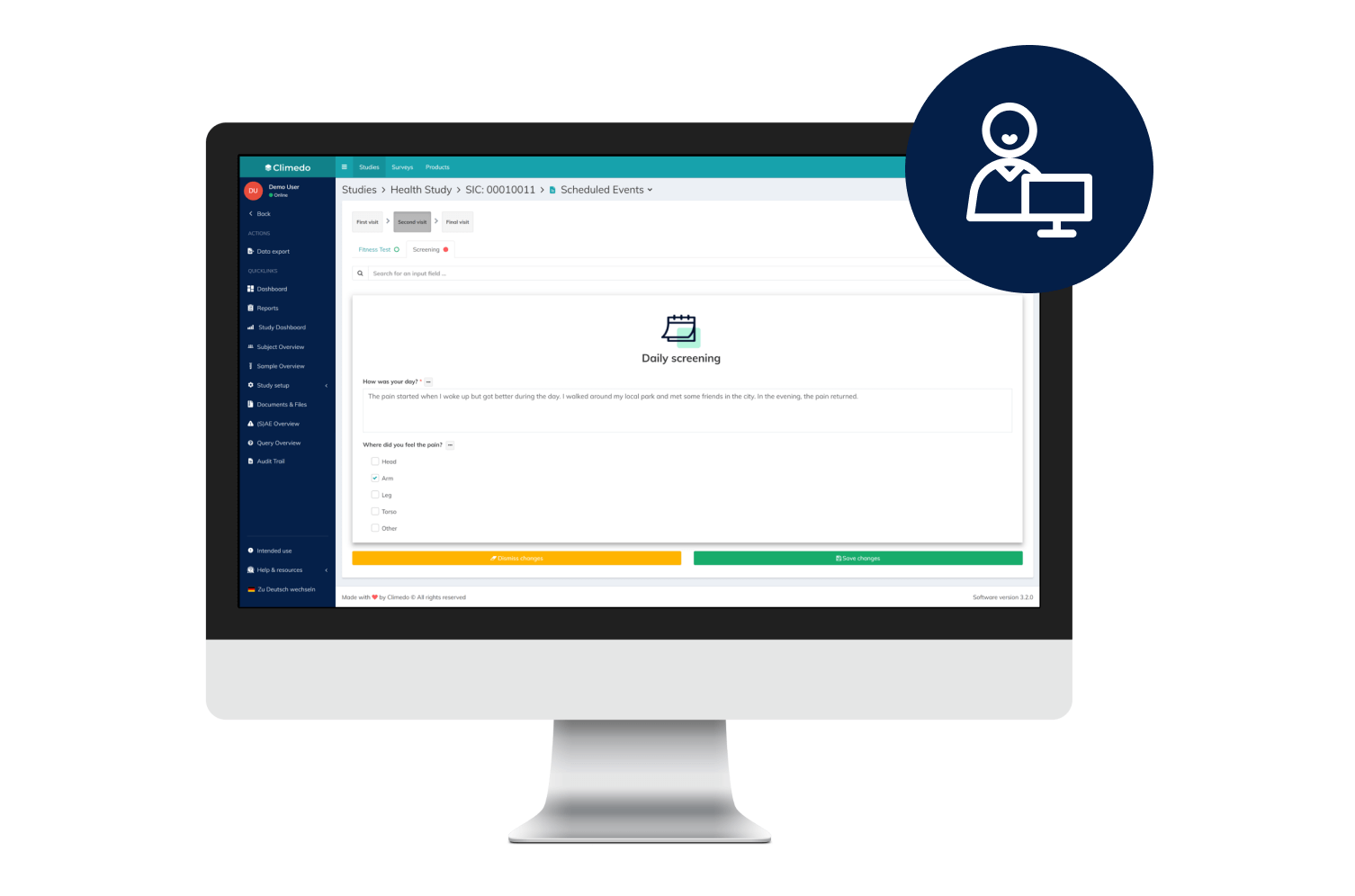
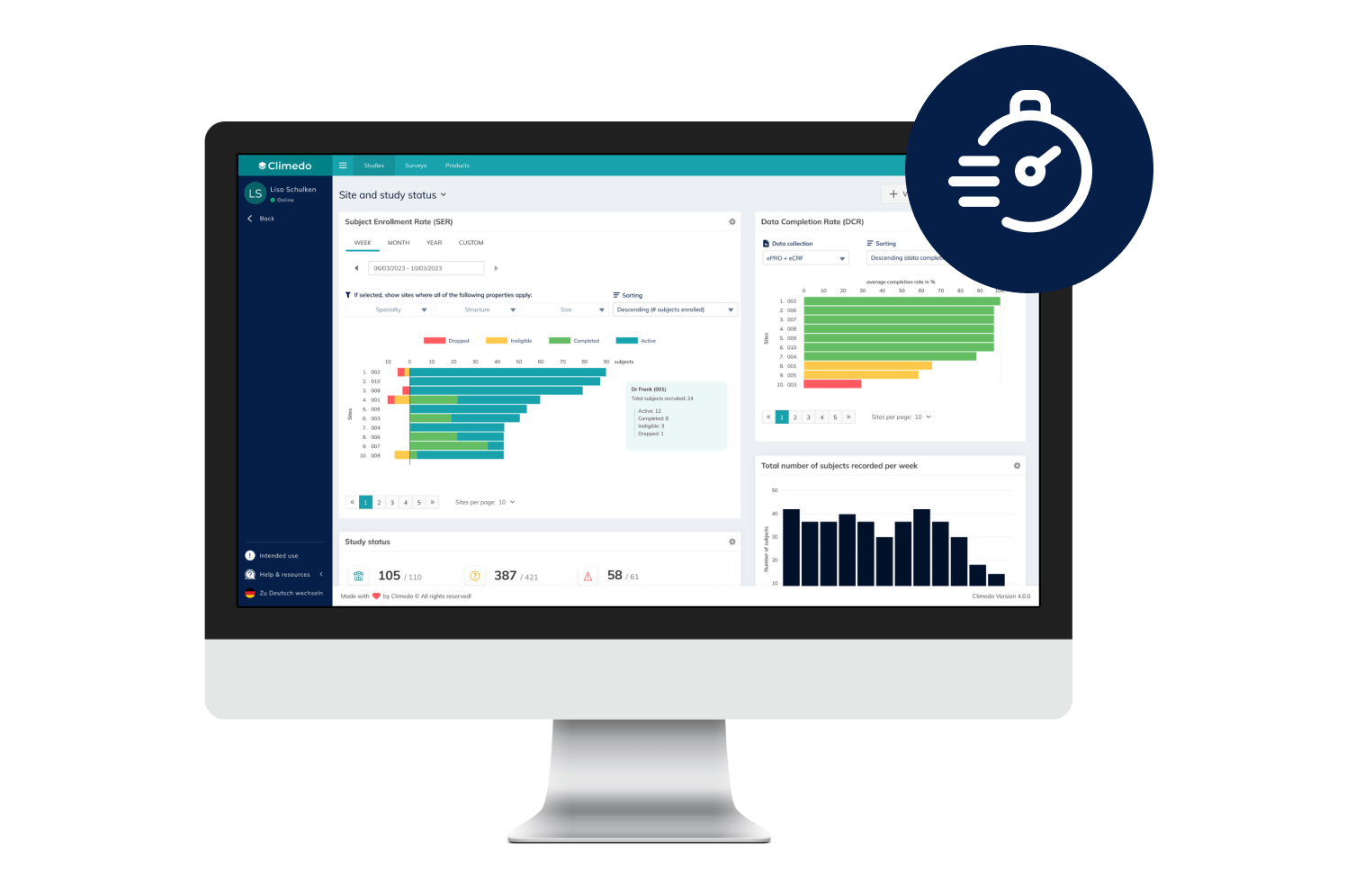
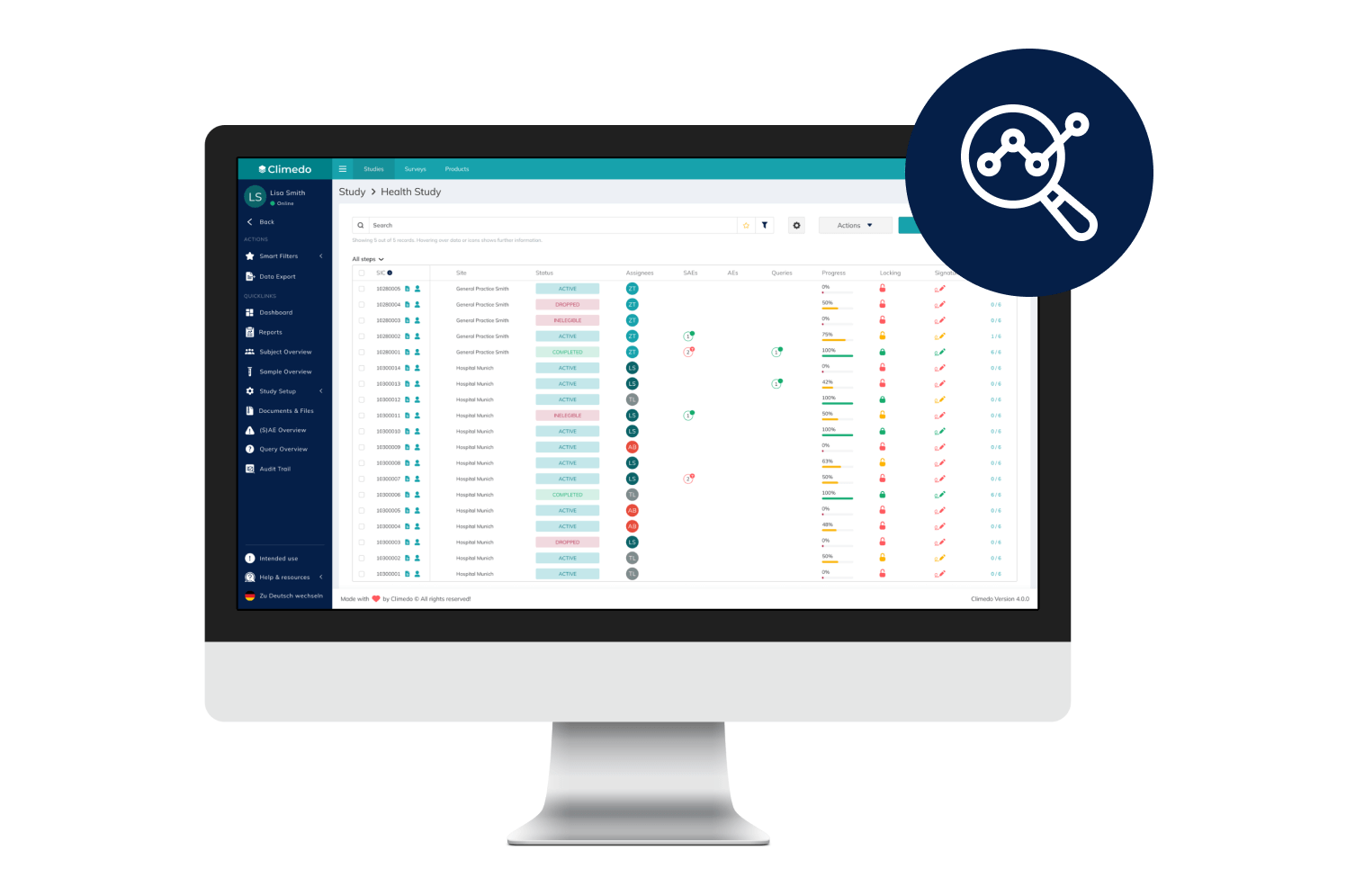
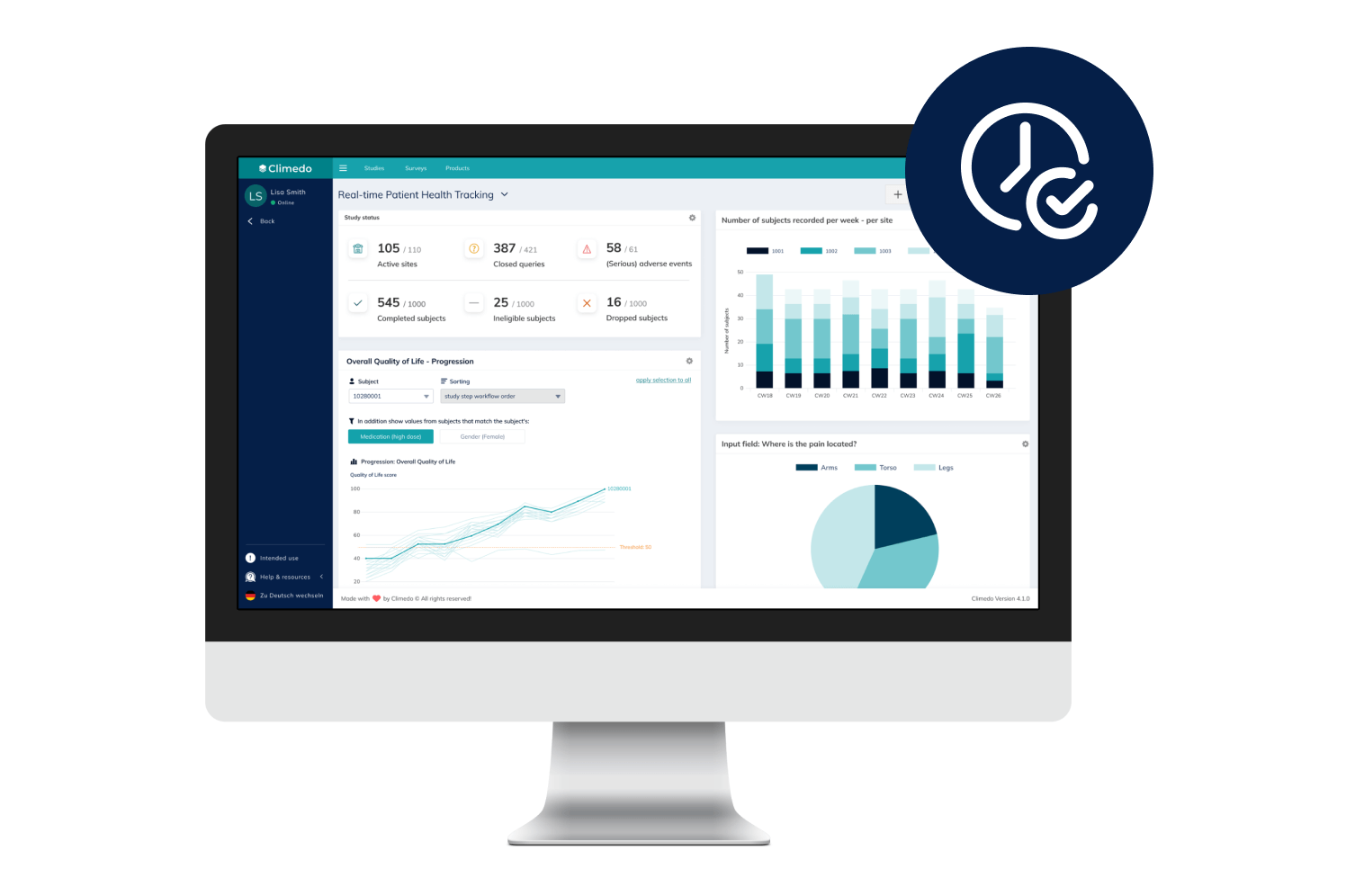
Climedo’s User-Friendly, Scalable and Efficient EDC System
With Climedo's Electronic Data Capture (EDC) system, you'll be perfectly equipped for the future of clinical trials. Connect all stakeholders within one platform and capture medical evidence on your pharmaceutical and medical devices more quickly and easily – cost-effectively, automatically and securely.