Climedo for Pharma and Biotech Companies
Faster Patient Access to New Pharma Treatments
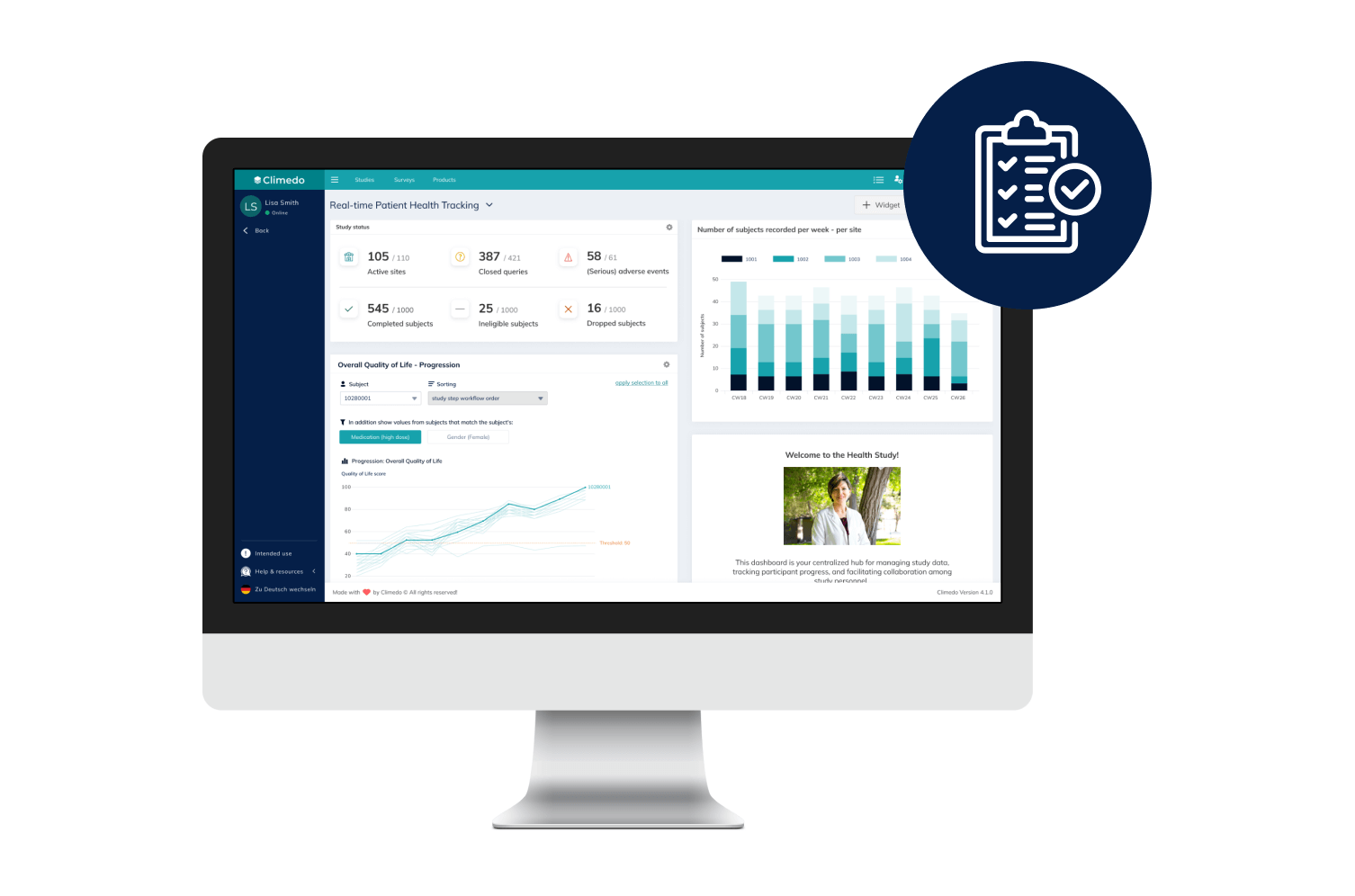
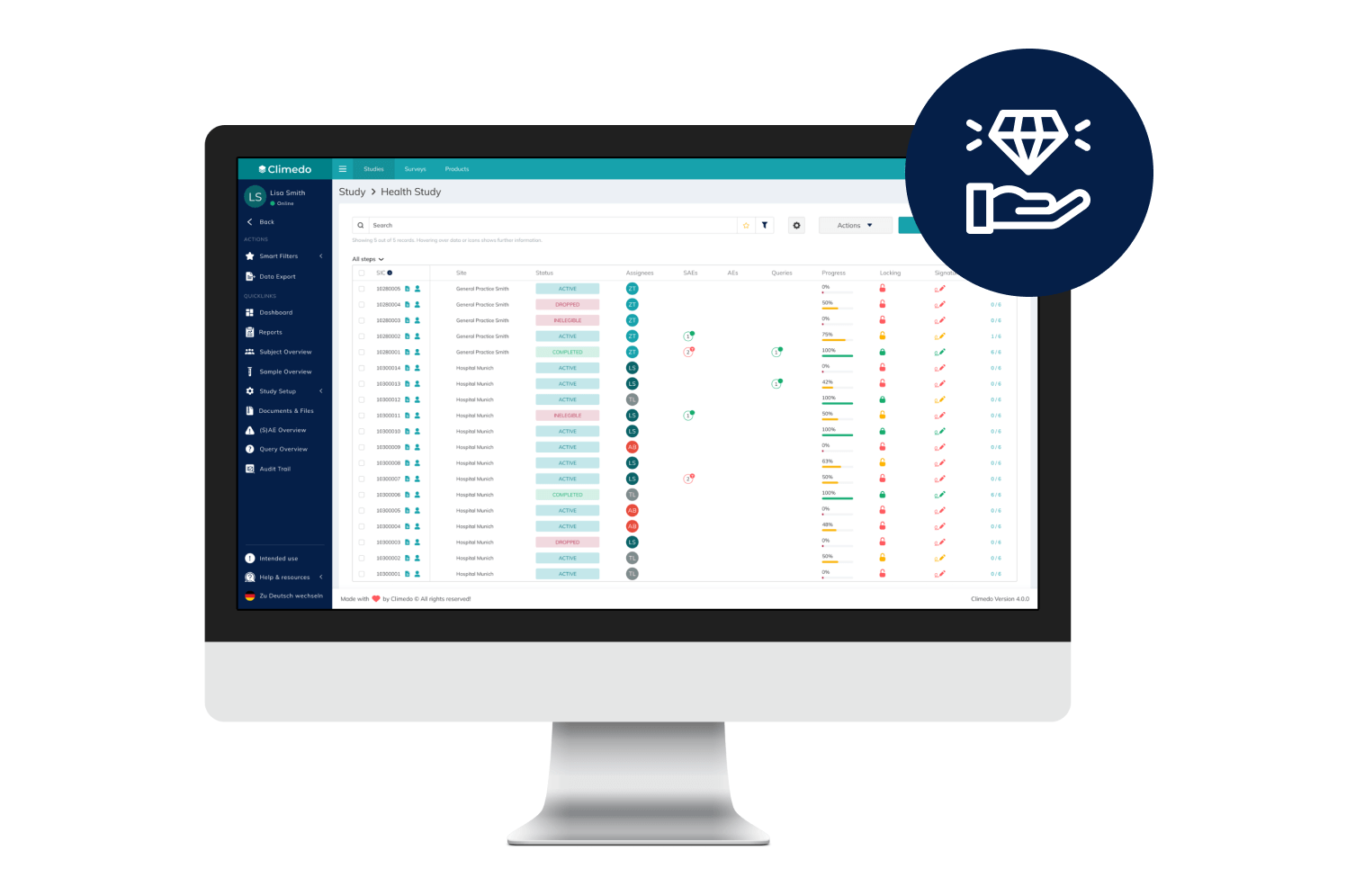
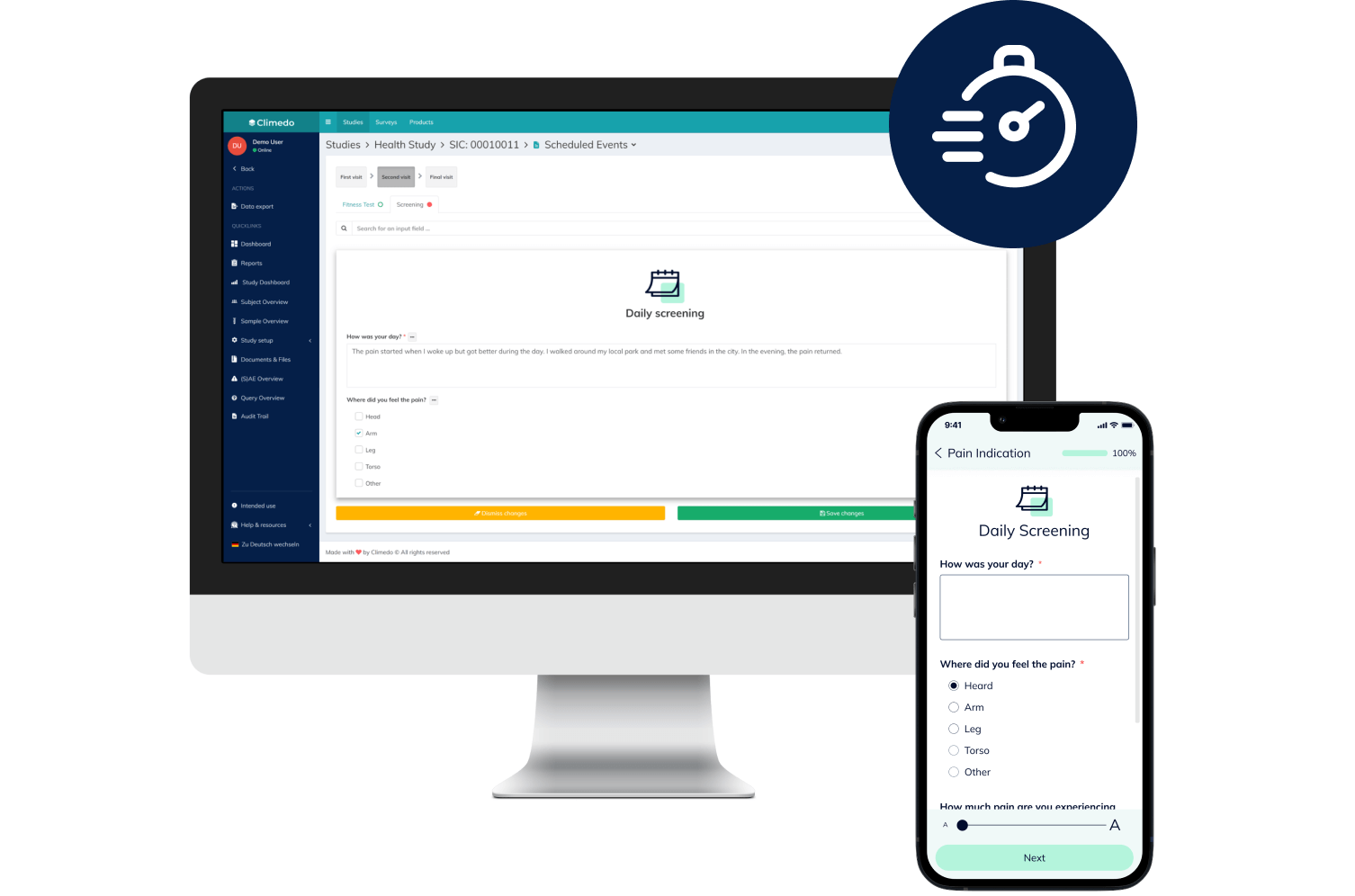
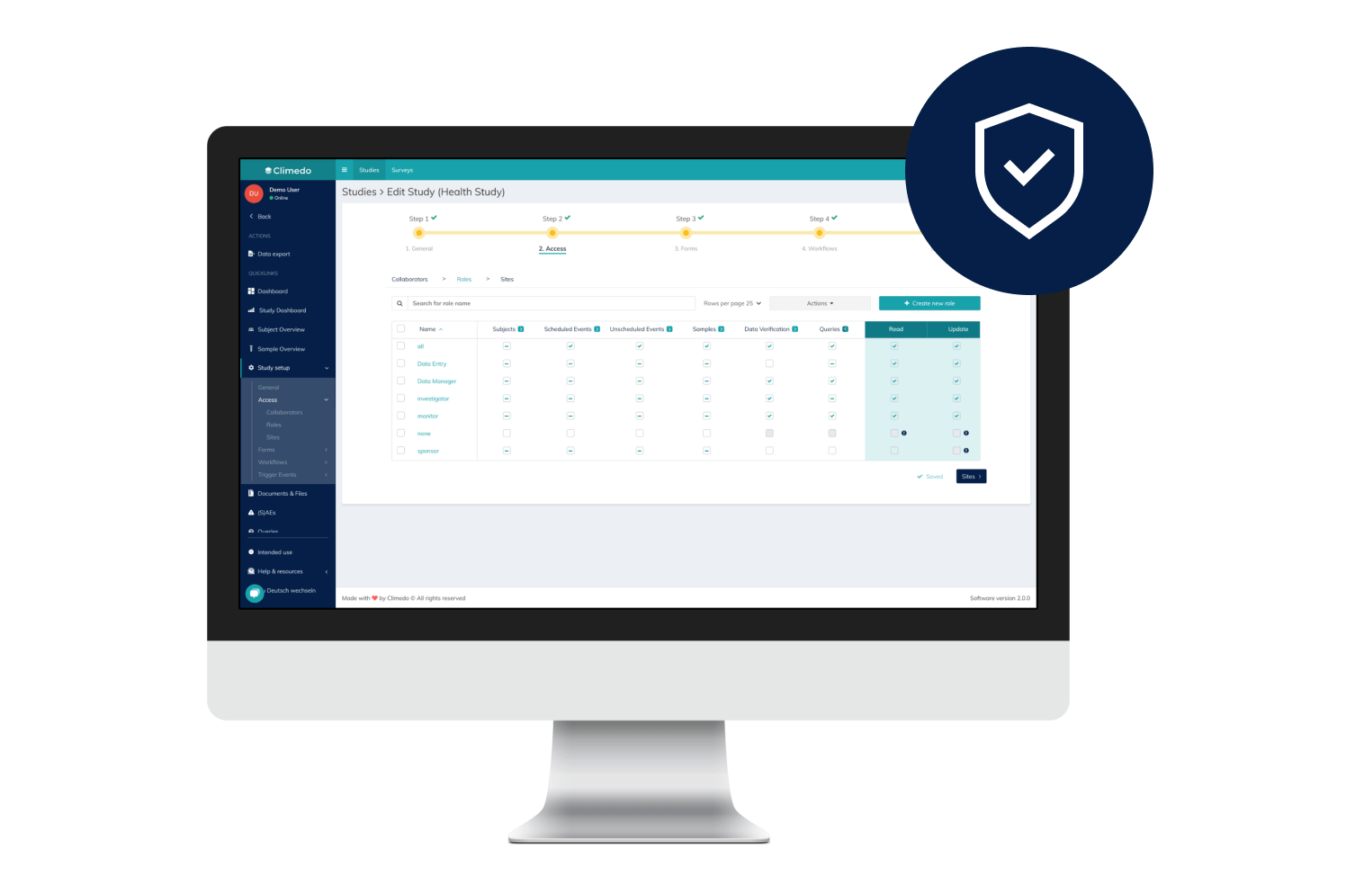
Offer your patients access to novel treatments 18-24 months faster and enhance medical visibility for HCPs early on. Optimize scientific dialogue with the support of real-time, descriptive dashboards while an RWE study is still underway. Our platform supports your pharma and biotech trials – from Phase I-IV to interventional or NIS to RWE.