Climedo for CROs
Faster Clinical Trial Success: The EDC Platform for CROs
Easily collect high-quality clinical data with our web-based platform. Save your CRO and your customers time and money.
Easily collect high-quality clinical data with our web-based platform. Save your CRO and your customers time and money.


Clinical data collection is time-consuming and costly. The requirements of increasingly complex study designs often lead to hardly scalable solutions that are expensive in quality assurance and project management. Monitoring activities lead to considerable expenses and heterogeneous data sets require a high cleaning effort. At the same time, cost pressure is increasing on the sponsor side.
With our web-based platform, you can quickly and easily collect high-quality clinical data. Save yourself and your customers time and money thanks to digital, reusable and automated workflows and the direct engagement of patients. In addition, our system supports you in data preparation for faster analyses. With Climedo at your side, you are prepared for the future of clinical trials and win more orders.

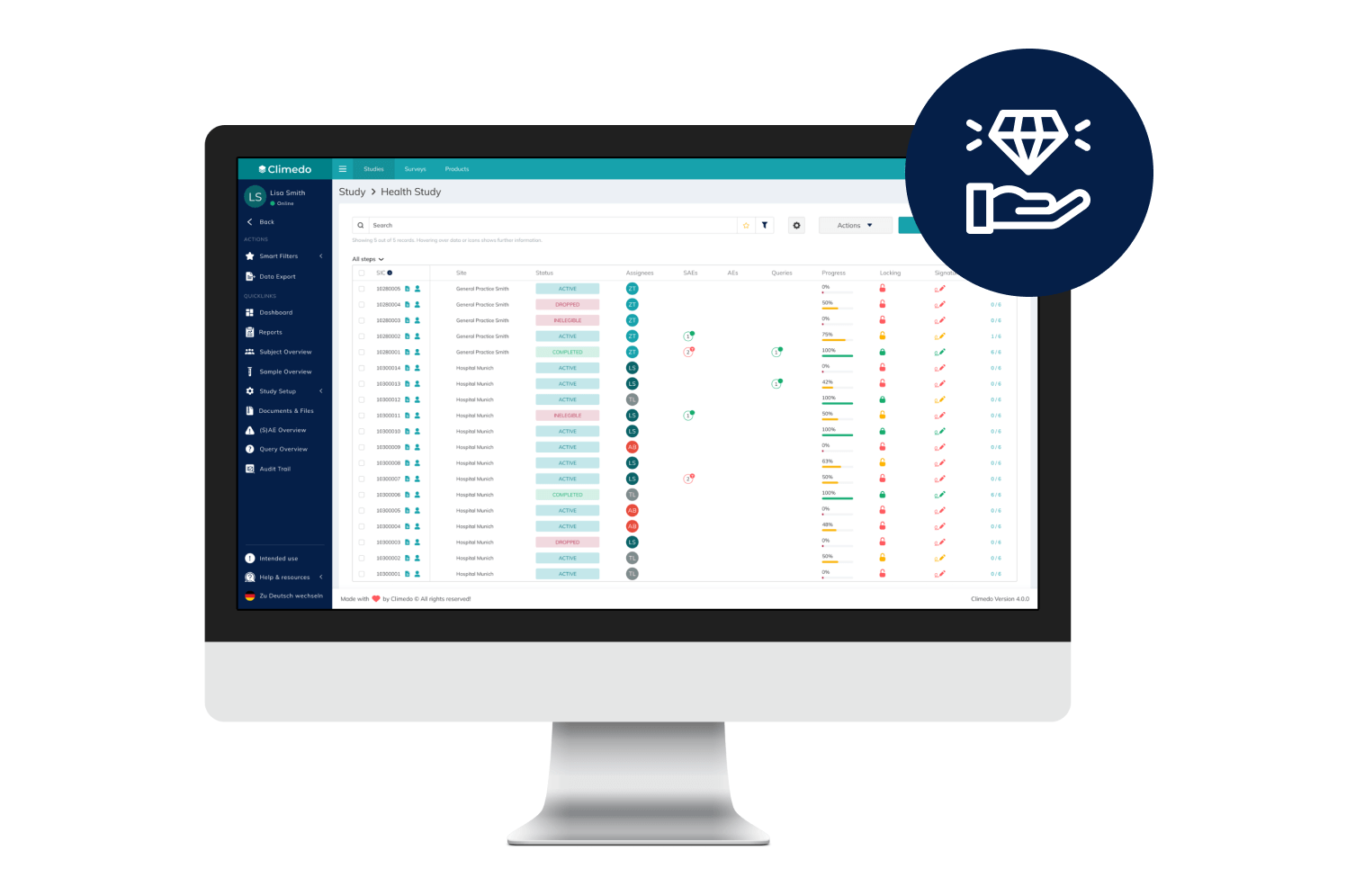
EDC
Cost-efficient, automated and scalable: Capture data for your clinical studies and surveys electronically with our EDC system – all in one place.
Our EDC System
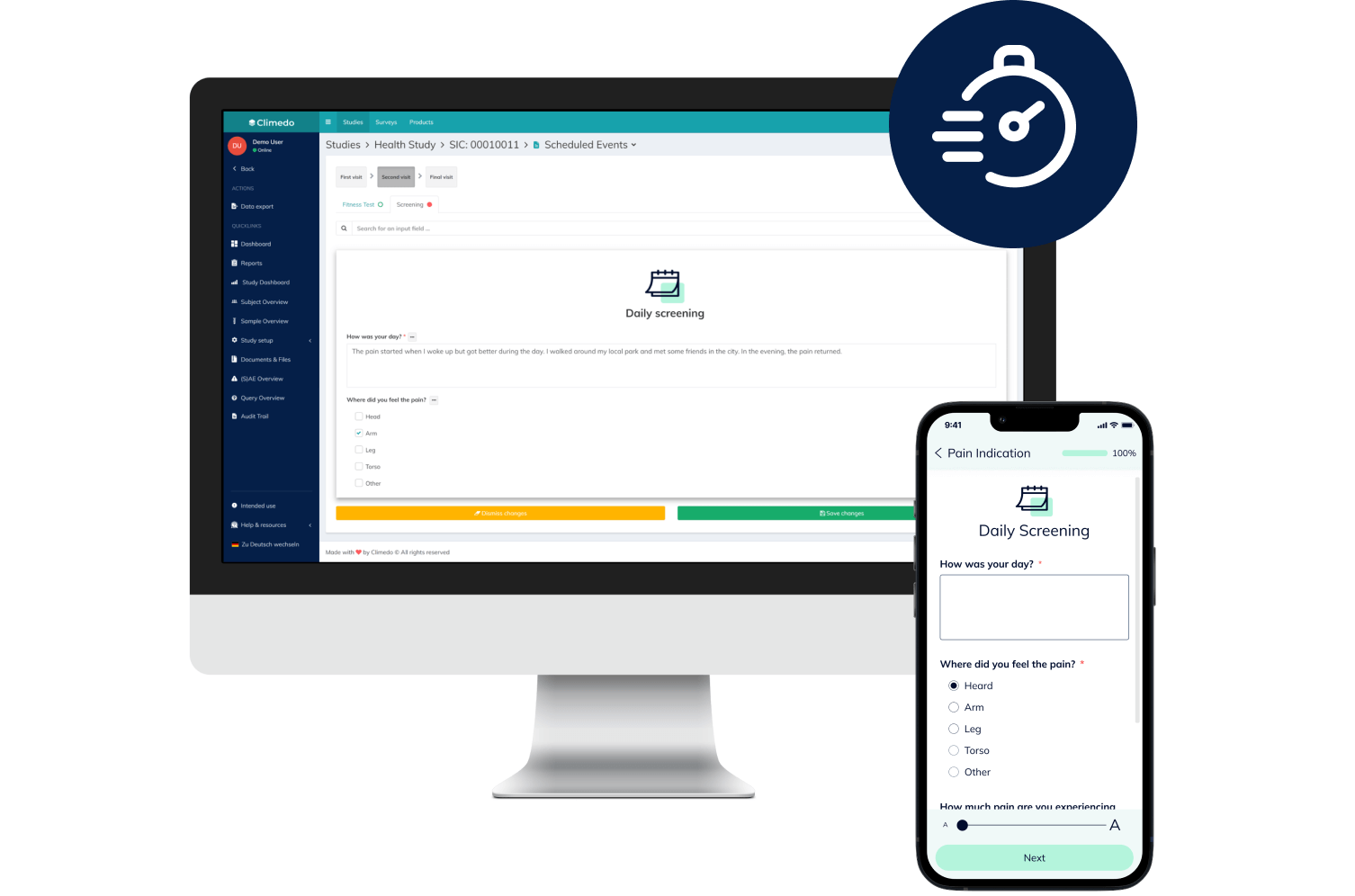
eCOA & ePRO
Convenient, secure and fast: Digitally capture all data reported by your patients in the course of a clinical study – on any device and with no app.
Our ePRO Solutions
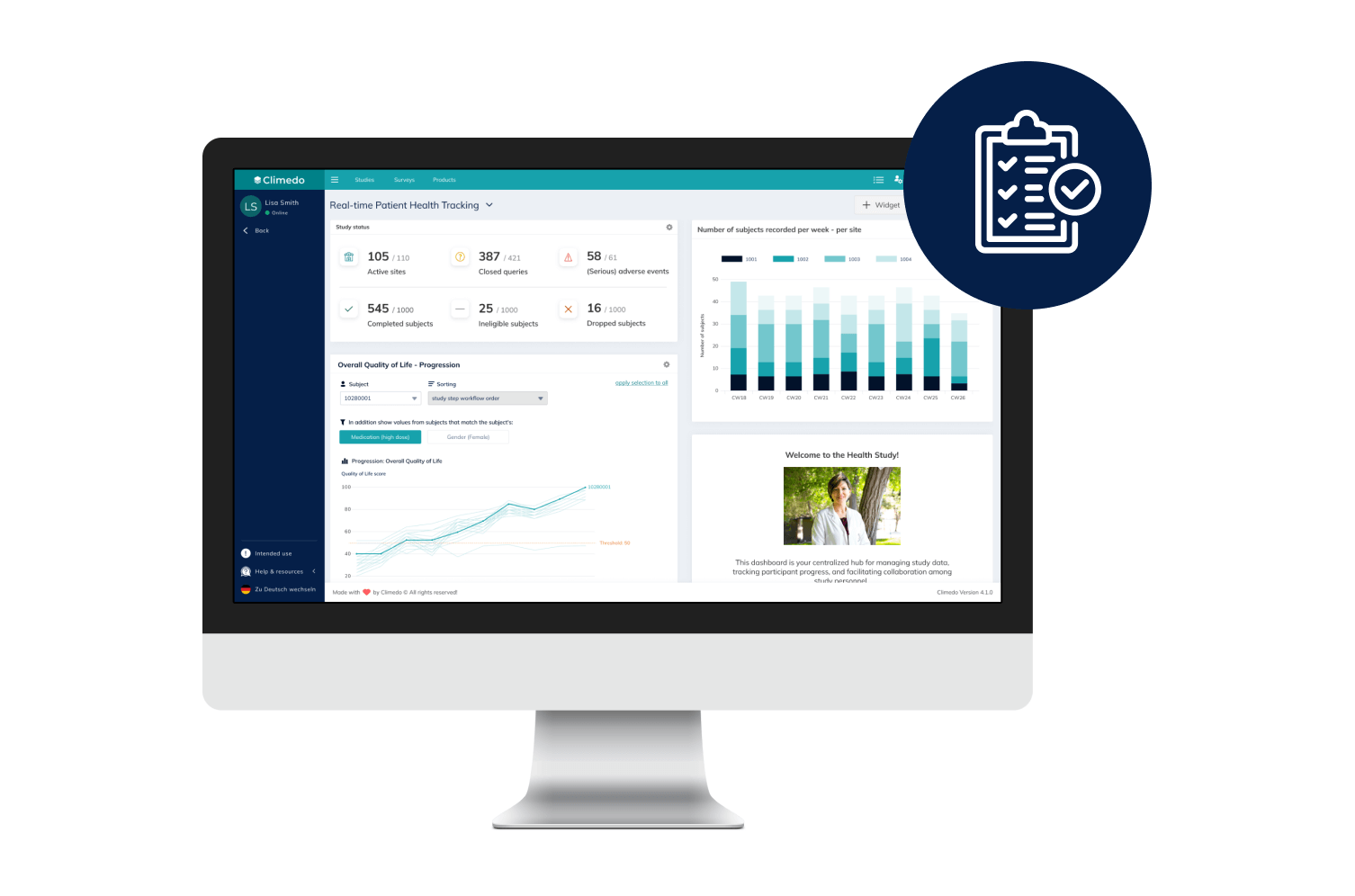
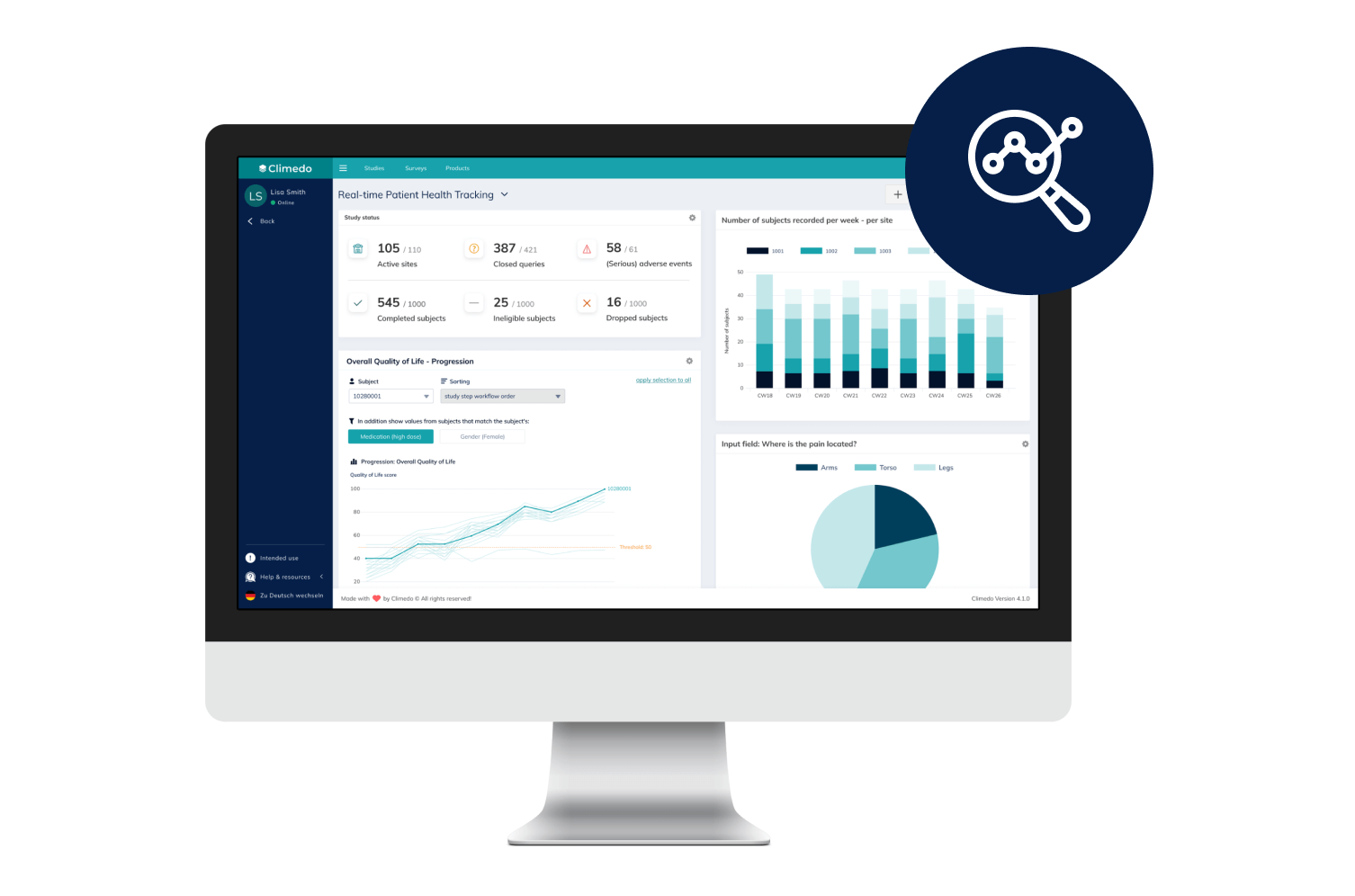
Real-Time Dashboards
Our interactive dashboards enable you to see real-time visualizations of your clinical trial data and compare patient cohorts across different sites.
Our Real-Time Dashboards
eConsent
Simplifies your administrative processes at the start of a study. Study participants are informed digitally.
Our eConsent
Hybrid Trials
Implement clinical trials in a patient-centered, flexible, and decentralized manner, during the pandemic and beyond.
Our Hybrid Trial Solutions

We'll show you how to start a clinical trial or survey and address your specific needs and desires.
Climedo's intuitive user interface means you'll be onboarded in just a few hours, without the need for programming skills. Get started with your project right away.
Patient centricity and decentralized study designs accelerate your recruitment and increase patient compliance by up to 90%.
Predefined workflows with complex decision trees including automatic plausibility and validation checks improve the data quality from the time of input.
Achieve significant time savings of up to 49% thanks to reusable form templates and the possibility of decentralized study designs and remote monitoring.
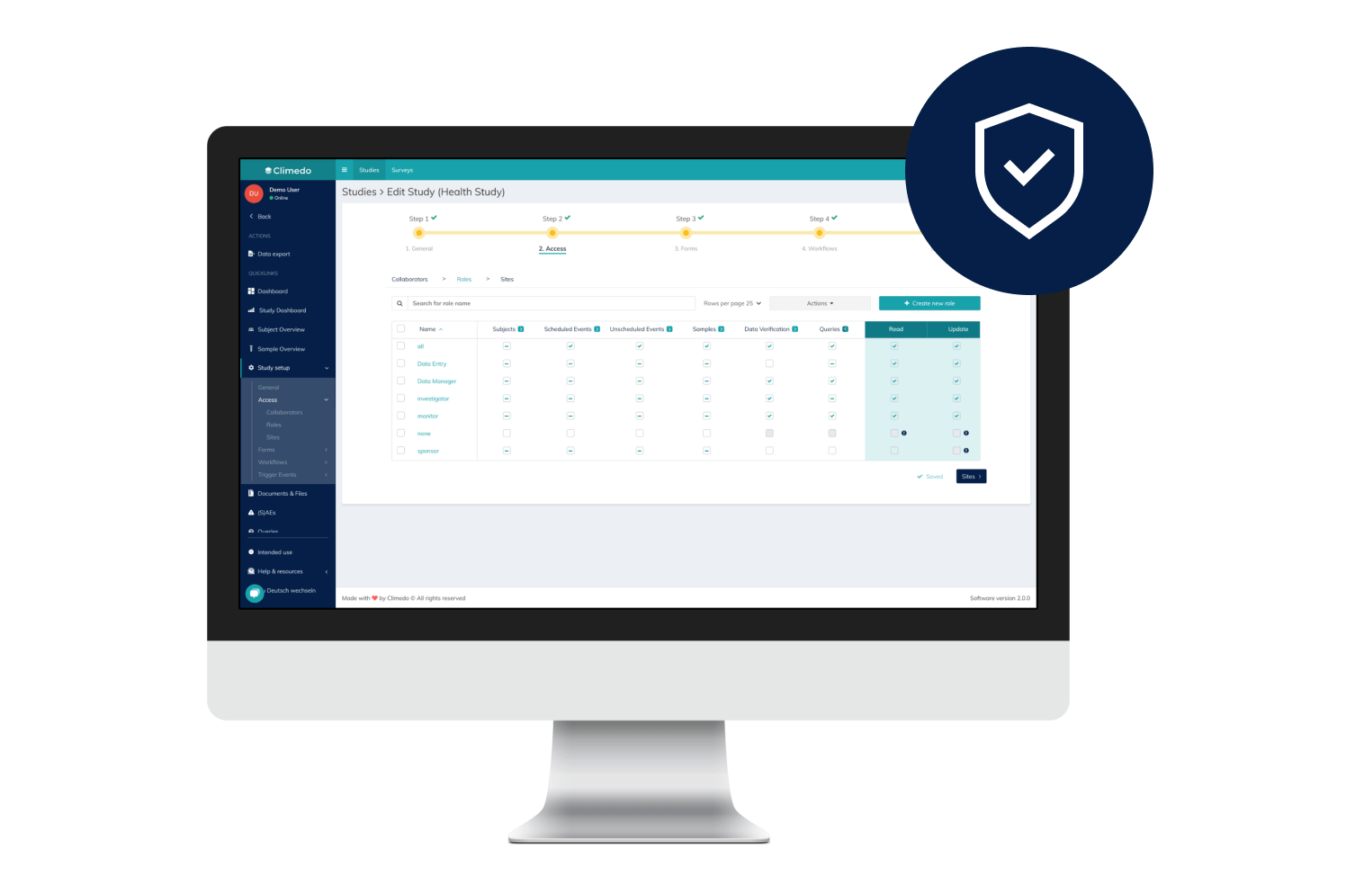
Our cloud-based solution has been tested by state authorities and offers a granular roles and rights assignment for different system users.
Dashboards and smart filter views allow you to view all results in real time and thus anticipate potential problems. Data exports are possible in common formats.

Dr. Jens Milde
Managing Director, Pharmalog Institute for Clinical Research
Felix Alt
Head of Clinical Operations, analyze & realize GmbH

Thanks to the simple handling (independent of location or device), the intuitive usage and the automated reminders, you'll see higher participation and completion rates.
Direct patient feedback and paperless data collection will improve your data quality. With Climedo, the error-prone transfer of paper to Excel sheets and illegible handwriting become a thing of the past.
Faster results thanks to automated processes, reusable forms and decentralized study designs as well as remote monitoring.
