Engaging KOLs in the Post-Approval Phase – 3 Expert Tips
DATE
November 21, 2023
AUTHOR
Veronika | Co-Founder & COO
Introduction
This article will discuss why KOL (Key Opinion Leader) engagement is so important in the post-approval phase on a clinical trial.
A Phase IV clinical trial, also known as a post-marketing surveillance trial or a post-approval study, is conducted after a new treatment (such as a pharmaceutical product or medical device) has been approved by regulatory agencies and has entered the market. It represents a pivotal phase in a product’s journey from development to market availability. Unlike earlier phases of clinical trials, which primarily focus on evaluating the treatment’s quality, safety and efficacy, Phase IV clinical trials are designed to gather additional information about the product’s real-world use, long-term effects and broader safety profile. This trial phase can be more complex and resource-intensive than earlier phases. Recruiting study sites or engaging patients and healthcare professionals (HCPs), particularly KOLs, in the post-approval phase can pose several challenges for study sponsors. In fact, KOLs are often considered as the most relevant stakeholder group. Regulatory requirements and the competitive landscape in the pharmaceutical industry further contribute to the complexity. Meeting these challenges therefore requires a strategic and well-coordinated approach by study sponsors.
By the way, if you want to find out more about mapping and prioritising KOLs, check out this post.
Which role do KOLs play in the post-approval phase?
KOLs in the post-approval phase are instrumental for pharmaceutical and medical device and launch. They contribute to the product’s credibility, educate healthcare professionals, offer holistic real-world insights and assist in data capture. Their expertise, influence and network connections contribute to the successful introduction and adoption of pharmaceutical products within healthcare systems, ensuring that patients have timely access to innovative treatments. Although KOLs play an important role in the success of post-approval studies, it can be challenging to engage them in meaningful scientific dialogue. In the following, we will share three tips for improving KOL engagement in post-approval clinical trials.
Whitepaper: From Data to Dailogue – Elevating Science-Based Communication in Post-Approval Studies
Our whitepaper shows how to gain an advantage in the post-approval phase. Explore the following topics:
- The challenges in post-approval studies
- How interactive digital tools can help address these challenges
- Real-world use case: Leveraging benchmarking and progression features

3 tips for KOL engagement:
1) Open communication and genuine relationships with KOLs in the post-approval phase
Face-to-face interactions with HCPs and KOLs in the post-market phase are indispensable, fostering trust and in-depth discussions. They don’t necessarily have to take place in person either, but can happen via a video call, for example. These interactions enable the exchange of specialized knowledge and expertise between Medical Science Liaison (MSL) managers or field workers from pharmaceutical companies and KOLs, improving clinical trial design and patient outcomes. KOLs possess invaluable market insights, making building personal relationships crucial. Such bonds humanize collaborations, enhancing loyalty and advocacy. Moreover, connecting with one KOL can create a network effect, facilitating interactions and positive word-of-mouth in the field.
Communication, trust and strong relationships are the linchpin in KOL engagement during post-market clinical trials. When based on real-world scientific evidence, these interactions foster knowledge exchange, provide access to market insights, nurture personal connections and create opportunities to engage with other KOLs. Acknowledging these factors is essential for lasting success.
2) Insights into new scientific discovery
By providing KOLs in the post-approval phase with exclusive access to new research findings in real-world settings, companies not only acknowledge their expertise but also involve them in the forefront of medical advancements. Sharing these insights fosters a sense of collaboration and positions them as crucial contributors to the evolving scientific landscape. This engagement can spark intellectual curiosity, encouraging KOLs to actively participate in Phase 4 trials and applying newfound knowledge to real-world patient care. Moreover, by recognizing and valuing their role in disseminating these discoveries, MSLs can establish a strong partnership that extends beyond the trial, enhancing the credibility of the sponsor’s research and ensuring the integration of innovative treatments into clinical practice.
Furthermore, the exchange of insights can lead to valuable discussions, enabling KOLs to provide nuanced perspectives on the practical application of these discoveries. This collaborative dialogue not only refines trial methodologies but also enhances the overall impact of Phase 4 research, aligning it more closely with the evolving needs and challenges faced by healthcare professionals. Another potentially valuable aspect is off-label drug use (OLDU), which is often not discovered until a Phase IV trial is conducted. In real-world practice, it is sometimes possible to discern new ways of using a drug (such as a new administrative route) or a new indication (such as low-dose aspirin as an anti-platelet agent). Sponsors should not use this data as part of a clinical trial but can apply to the regulatory body to conduct a formal study in this new indication – which the KOL could then be involved in at a later stage. This helps medicine to advance, a further motivating factor for HCPs.
Climedo Connect: Proven Strategies for Dynamic Commercialization in Europe’s Pharma Landscape
In this Climedo Connect Catarina Santos (Executive Director Region Europe, Novartis) and Veronika Schweighart (Co-Founder & COO, Climedo) presented success factors for navigating product launches and commercial success in Europe’s pharmaceutical landscape.

3) Real-time data insights for KOLs with interactive dashboards
Interactive dashboards are vital tools for driving KOL engagement during the post-approval phase of clinical trials. They offer real-time data access, personalized insights and visual representations that simplify complex information. This supports KOLs in staying informed, making timely decisions and effectively conveying data to HCPs and the public. Efficiency is another key benefit, as high-quality dashboards streamline data analysis and facilitate quick filtering and thereby access to specific patient groups and their real-world response to a treatment. Additionally, these dashboards support collaboration and communication among KOLs and other stakeholders, promoting knowledge sharing. KOLs can track their own contributions and impact through interactive dashboards, encouraging continuous improvement.
One example for this are Climedo’s Benchmarking and Progression features in post-approval studies.
How are specific study subjects doing with regard to certain lab parameters compared to the overall study population? Which differences can be identified for different subgroups? These are just some of the questions which are often asked by KOLs and sponsors. Thanks to Climedo’s Benchmarking and Progression features, KOLs and sponsors are supported in visualizing and evaluating the captured results.
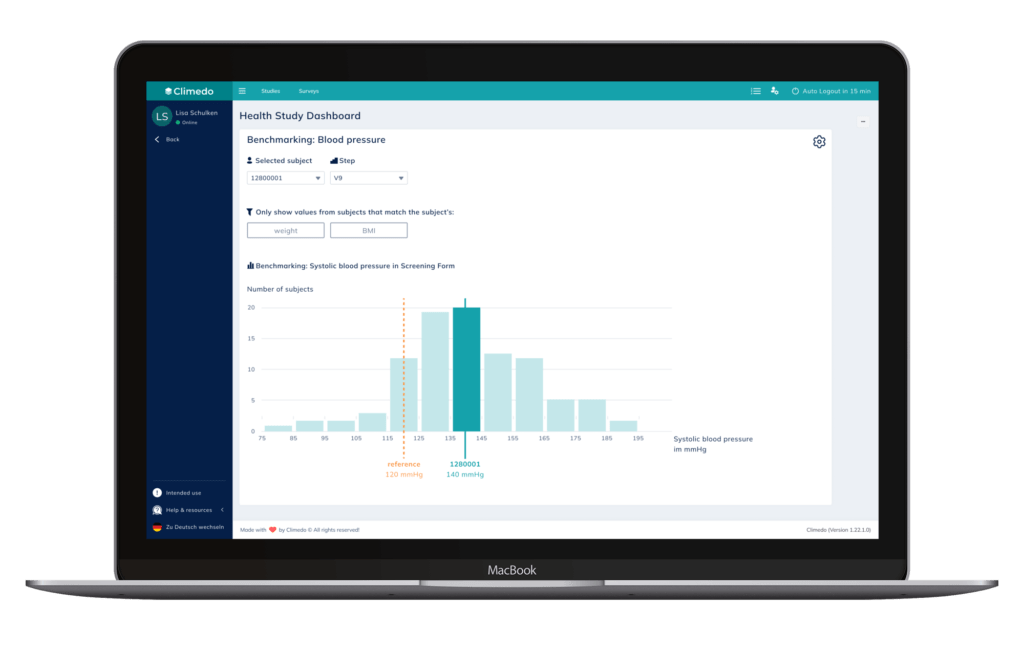
First, the Benchmarking feature allows HCPs to compare a defined data point of a subject to the overall, anonymous study population. Optionally, MSLs could also be equipped with such tools to inform new KOLs about the study, bearing in mind that the data is not yet representative.
In the example shown below, data can be filtered by body weight and BMI. The treating physician can select the treated patient and thereby highlight their positioning in the histogram relative to the selected (sub)population. Data is compared for a given visit (“step”), e.g. data from the 9th visit, so that the treatment duration is comparable among the displayed, anonymous patients.
The Progression shown feature below, on the other hand, enables users to see the trajectory of a patient’s selected parameter, captured over a specific time period (such as multiple site visits).
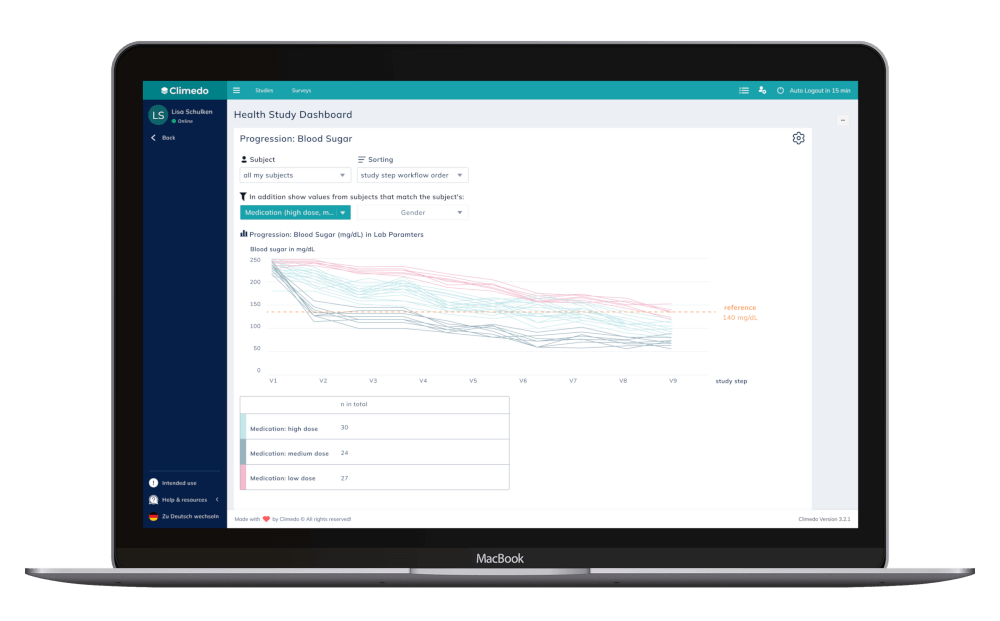
Imagine that a patient with diabetes is participating in a clinical study for insulin. Within the Climedo dashboard, the treating physician can view their progress based on their blood glucose level measured in the morning before eating or consuming sugar-containing beverages, and track how their values compare to the overall study population and within certain weight or BMI classes. This visualization improves transparency about study progress for the KOL and helps them detect potential issues early on, thus benefiting the patient and improving engagement in the study.
Furthermore, modern dashboards are accessible from various devices, enabling KOLs to engage with data and collaborate regardless of their location. In summary, interactive dashboards empower KOLs to make informed decisions, communicate effectively and contribute significantly to the success of clinical trials and the responsible promotion of medical products.
Conclusion
The post-market phase in clinical trials is a critical juncture in the journey of pharmaceutical products and medical devices from development to market availability. It involves unique complexities and challenges, particularly in engaging KOLs effectively. These influential figures play a pivotal role in the credibility, education and success of post-market studies, making their engagement paramount. By implementing the above-mentioned tips and strategies, study sponsors can enhance KOL engagement, ultimately contributing to the success of post-approval trials and the responsible promotion of medical products. In this dynamic landscape, acknowledging the importance of these approaches is essential for lasting success.
Want to learn how to enhance KOL engagement in your next clinical trial with the help of digital technologies and real-time data insights? Feel free to schedule a demo with us!