CRO Workshop Event: Exploring the Future of Patient Centricity in Clinical Trials

DATE
August 01, 2023
AUTHOR
Tereza | Senior Customer Success Manager
In an ever-evolving healthcare landscape, embracing patient-centric approaches in clinical trials is crucial. That’s why, in early 2023 we organized a thought-provoking CRO workshop titled “The Future of Patient Centricity in Clinical Trials.” The event aimed to foster innovative discussions around the present and future of clinical research, and to establish new connections within our CRO network.
What was the CRO workshop about – Quick overview:
1) Part 1: Futures thinking
1a) Clinical trials in the metaverse
1b) Personalized medicine through AI and wearable devices
2) Part 2: Data insights
3) Customer engagement and feedback
1) Part 1: Futures thinking method
The event kicked off with an introduction by our Customer Success Manager Lena, providing an overview of the workshop’s objectives. Hannah, our Product Manager, then introduced attendees to the “Futures Thinking” method, which would serve as a framework for envisioning patient-centricity in the next 15-20 years. It is crucial to clarify that Futures Thinking does not aim to predict the future. Instead, it provides a framework that fosters our imagination to anticipate and prepare for the future. It helps us analyze emerging trends, anticipate customer needs, and adapt our strategies. By envisioning different scenarios, we can make informed decisions, innovate, and stay competitive in a changing world. Futures thinking empowers us to shape our own future, be strategic, and plan ahead.
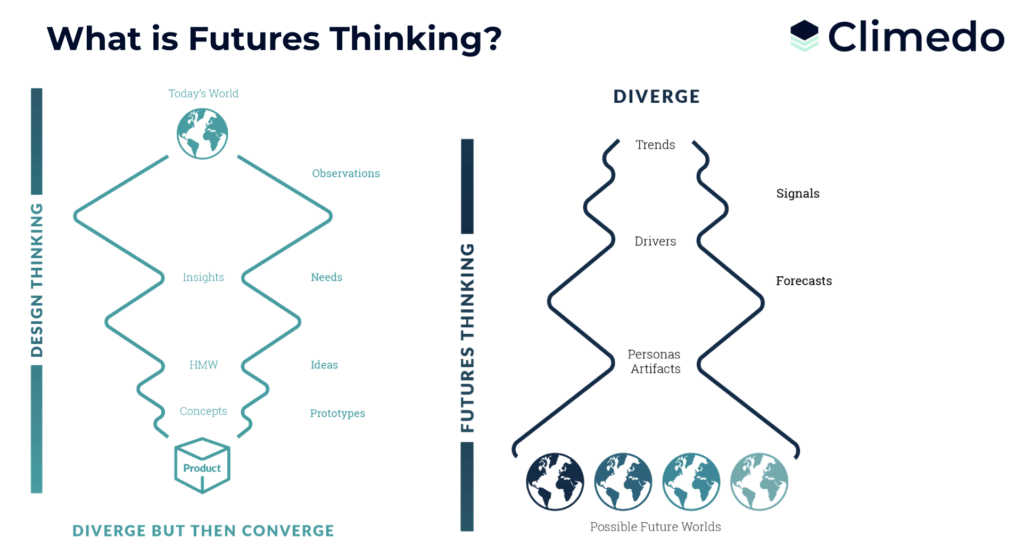
1a) Clinical trials in the metaverse
One group in the workshop explored the potential of the “Metaverse” as a platform for medical care. Their findings highlighted exciting opportunities, including easier patient recruitment, access for individuals in remote areas, improved basic care, faster data sharing and even the possibility of virtual therapies. However, challenges such as confidence in Metaverse technologies, data misuse risks, regulatory adaptations and potential competition from big tech companies must be addressed. The group posed intriguing questions, e.g. about the role of CROs in the Metaverse, potential partnerships with data providers, the need for a GCP (Good Clinical Practice) for the Metaverse and the impact on study population diversity.
1b) Personalized medicine with AI and wearable devices
The second group focused on the acceptance of smart devices and the use of AI in enabling personalized medicine. They explored the potential benefits, including better prevention, personalized diagnostics, enhanced patient monitoring, increased life expectancy, resource savings and improved benefit-risk profiles. The group also pondered questions about the regulatory landscape, the balance between AI and human involvement and strategies for generating market acceptance for new technologies.
2) Part 2: Data insights outcomes
Following the Futures Thinking sessions, our attendees engaged in a data insights workshop facilitated by our COO Veronika and our Pharma Lead Matthias. They presented use cases and encouraged brainstorming and discussions. Customers provided valuable feedback and told us more about the features that would help them make their clinical trials even more efficient.
3) Customer engagement and feedback
The CROs at the event demonstrated a commendable spirit of collaboration, setting aside competitive dynamics in favor of shared learning and progress. They were highly engaged and enthusiastic throughout the workshops and participated actively. The event received exceptional ratings, with attendees expressing high satisfaction with the overall experience. This success encourages us to explore concrete use cases and detailed discussions further in the future.
We hope you enjoyed the insight into our CRO workshop event. Would you like to get a better idea of our solutions for clinical trials? Feel free to schedule a short demo and to talk to one of our experts. We look forward to hearing from you!






