Leveraging Real-World Evidence for Accelerated Commercialization

DATE
September 13, 2023
AUTHOR
Catherine | Associate Director Marketing
Real World Evidence (RWE) plays a pivotal role for pharmaceutical companies in optimizing market access and commercialization of new drugs. It enables stakeholders to anticipate and meet the expectations of external parties throughout a pharmaceutical product’s lifecycle. This blog post delves into the value proposition, terminology, data sources, critical success factors and new developments in RWE. By addressing these key aspects, pharmaceutical professionals can effectively leverage RWE to make informed decisions for clinical trial conduct, support payer decisions, enhance visibility and transparency of the new therapy’s impact, and ultimately drive commercial success..
The Value of RWE
RWE offers distinct advantages relative to traditional Randomized Controlled Trials (RCTs) across a product’s entire lifecycle. Unlike RCTs, which are conducted under controlled but narrowed conditions, RWE provides broad, end user-representative insights into the effectiveness and safety of treatments across diverse patient populations, not to forget the patient perspective itself in leveraging electronic clinical outcome assessments (eCOA). By considering real-world data (RWD) collected outside of clinical trials, RWE complements RCTs and fills knowledge gaps, allowing stakeholders to make more informed decisions about pharmaceutical products.
Terminology and Stakeholder Perspectives
Understanding the terminology used in the realm of RWE is crucial for effective communication and collaboration. Distinguishing between Real World Evidence (RWE) and Real World Data (RWD) is essential. While RWD refers to the raw data collected from various sources, RWE encompasses the insights derived from analyzing and interpreting that data. RWD often includes electronic clinical outcome assessments (eCOA) which, in turn, encompass four elements:
- Patient Reported Outcome (PRO) – comes directly from the patient
- Clinician Reported Outcome (ClinRO) – assessed by a trained healthcare professional
- Observer Reported Outcome (ObsRO) – performed by an observer (a non-clinician)
- Performance Rated Outcome (PerfO) – based on a task(s) performed by a patient
Think Global, Act Local: Best Practices for Holistic Real-World Evidence
Paul Petraro (Boehringer Ingelheim) shared valuable insights into the entire evidence generation process in one of our past Climedo Connect webinars. The following topics were discussed:
- How to strategically think about RWE generation globally and locally
- How to generate evidence in a holistic way
- Some best practices in the field of RWE from Boehringer Ingelheim

Additionally, level of detail (and thereby complexity) and communication mode (channels, language, style, among others) should be tailored to the specific needs of regulators, payers, physicians, interested patients and the pharmaceutical industry as a whole.
Data Sources and Robustness Assessment
Various data sources contribute to the generation of RWE, including electronic medical records, claims data, patient registries, pragmatic studies, observational studies, surveys and also healthcare wearables/sensors and even social media. Assessing the robustness of these data sources is crucial to ensure the reliability and validity of RWE studies. Rigorous evaluation criteria should consider factors such as data completeness, quality, representativeness, data significance and associated statistical analysis and potential biases.
Critical Success Factors in RWE Studies
Defining the right research questions, engaging stakeholders and considering alternative approaches are fundamental. Identifying the most robust and accessible data source, designing a robust data generation methodology and adhering to best-practice guidelines are also vital. Finally, pre-specifying a robust statistical analysis plan and effectively communicating RWE findings to different stakeholder types are additional success factors that enhance the credibility and impact of RWE studies.
The Next Frontiers ‘Live RWE’:
The field of RWE is continually evolving, with new developments and methodologies enhancing its potential. Machine learning and predictive analytics hold promise for predicting outcomes based on real-world data. Causal inference methods allow researchers to assess the effectiveness of drugs in real-world treatment populations, enabling a more comprehensive understanding of treatment outcomes. These advancements open up new avenues for leveraging RWE in optimizing market access strategies.
Climedo helps companies revolutionize RWE by offering interactive solutions that unlock the power of real-world data. This enables pharmaceutical and medtech companies to harness the potential of RWE in two transformative ways:
- Within the post-market study, Climedo provides a purpose-built toolkit that helps participating physicians to better contextualise and benchmark their treatment while ensuring regulatory compliance. This interactive approach enhances transparency and collaboration, generating valuable insights for stakeholders.
- Beyond the post-market study, Climedo equips Medical Science Liaison professionals (MSLs) with an up-to-date, interactive discussion basis for descriptive observation. This real-time information sets MSLs apart thanks to dynamic insights.
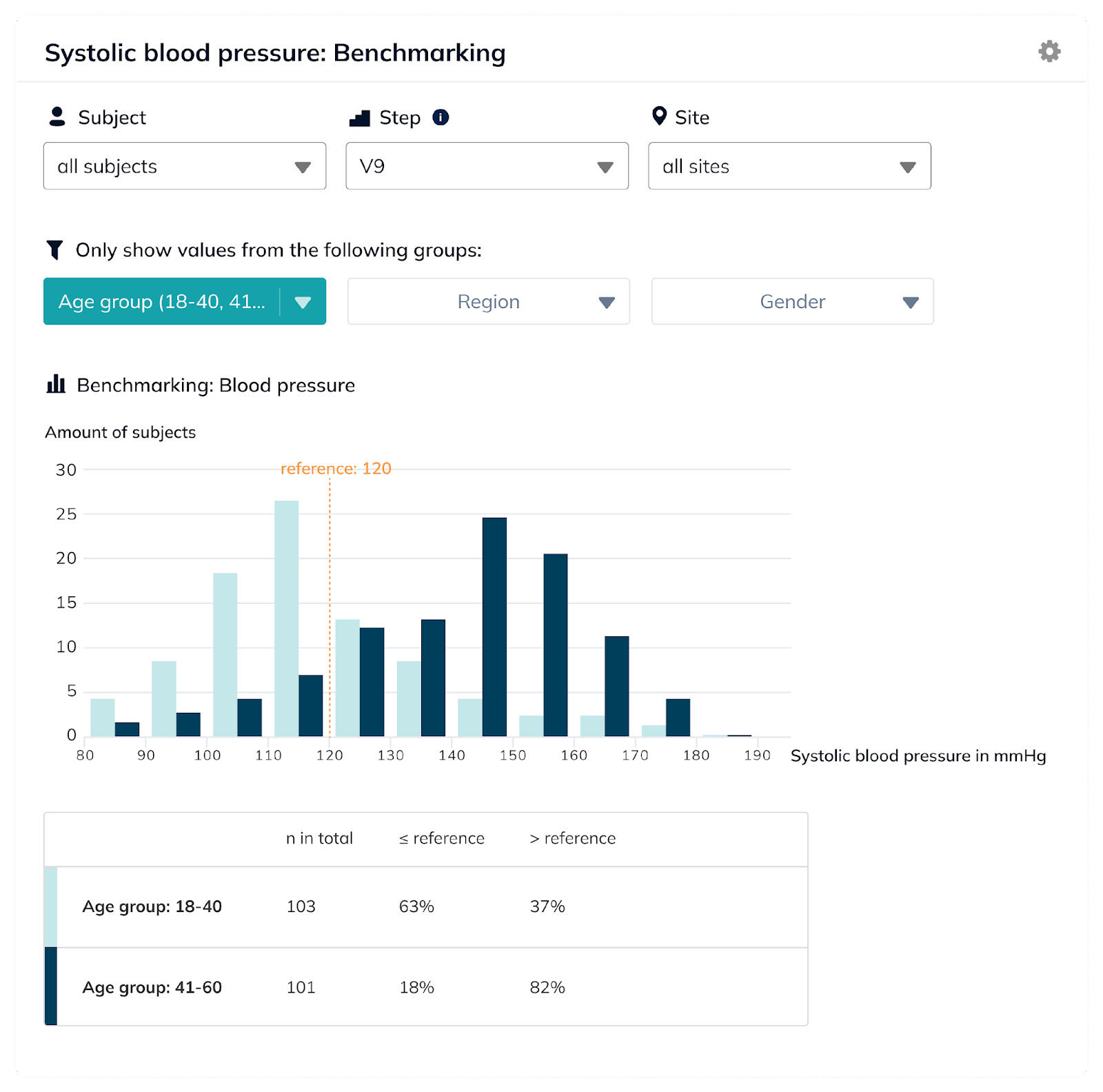
When used in the right way, this data is even more up to date than available reports, and, at the same time, it allows the pharmaceutical or medtech company to communicate their RWE even earlier. This accelerates commercialization and overall success of the new therapy or method.
Conclusion
Real World Evidence (RWE) is a critical area of expertise for pharmaceutical companies seeking to optimize market access. By grasping the concepts, methodologies and research techniques of RWE, professionals can anticipate stakeholders’ expectations and develop successful holistic RWE studies. Understanding the value RWE adds over RCTs, differentiating between RWE and RWD, assessing data sources, and considering critical success factors will empower pharmaceutical professionals to effectively leverage RWE throughout a product’s lifecycle. Furthermore, staying abreast of new developments and embracing emerging technologies will ensure that RWE remains a powerful tool in the pursuit of optimized market access and improved patient outcomes.
Want to learn how to effectively use RWE to accelerate commercialization for your company? Drop us a line or schedule a demo.