Another Exciting Software Update for Climedo: New Product Features Q3-2021

DATE
July 28, 2021
AUTHOR
Dragan | Co-Founder & CTO
Already using Climedo for your clinical data capture or simply want to learn more about our platform? In this post, we’ll present you with the new features which have been added since our last update!
What’s it about? – Quick overview:
- Custom notifications
- Additional Features: Slider input field (Visual Analogue Scale (VAS)), Hierarchical signatures, Display of assigned users, Select input fields for reuse
Custom notifications
Meaningful studies require a large number of subjects and with this amount of data it is often not possible to keep track of everything with conventional data collection methods. Therefore, not only is the central collection of data a great advantage of electronic data capture (EDC), but also the possibility of programming software in such a way that it interactively cooperates and always automatically informs you about the most important updates. Fortunately, with Climedo you don’t have to start programming any software yourself and you can set up which notifications you or your study staff should receive with just a few clicks. In our latest software update, the focus is on custom notifications, as we want to give our users the option to be notified themselves or to notify others when something happens in their study or survey that could be of particular interest to them. In addition, our users get an important feature to monitor the status of patients and the activities within the study more efficiently. Since the Q3 software update, it’s possible to set custom notifications for general events, form-specific events and field-specific events.
General events
A general event would be, for example, a (serious) adverse event ((S)AE)) or when all data for a patient become available. In addition, this is the case when an input form is locked/unlocked or signed, the (processing) status of a patient is changed, or queries have been entered. Notifications with customizable message texts and recipients can now be created for all these events.
- Locking and unlocking input forms: You can now be notified when an eCRF is locked for processing or when the lock is dropped.
- Queries: In our last software update, query management was a central topic. With the new changes, our users can now define more granularly which user is notified for which query events. Additionally, the notification message itself can be customized.
- (S)AEs: It’s crucial to keep an eye on the serious adverse events mentioned above, so that your study managers and medical study staff can take action as quickly as possible. For this purpose, you can now also set up more granular notifications that immediately inform selected users or user groups when an AE or SAE has been created, updated, signed, or archived.
- Signatures: You can also create a general event when a signature has been dropped or a new signature has been captured.
- Patient data: Another great update is the notification for patient data. This allows you to be notified when all required data are complete for a patient, a certain number of patients reach 100% completion status per study center or across the entire study, or when a patient’s status otherwise changes (e.g. when a patient drops out of the study, does not meet the inclusion criteria, etc.).
How to create a new event
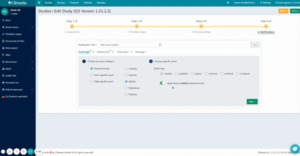
Let’s say you want to be notified immediately when a serious adverse event occurs in your study: You can now set up individual notifications in your study by editing your study in Climedo and creating an event for exactly this in the Notifications step. You can name it accordingly and additionally select that you only want to be notified in case of serious adverse events (see figure 1).
Fig 1. Adding individual notifications during study processing here using SAEs as an example
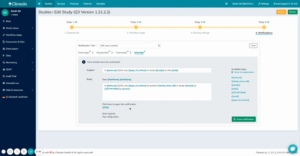
In the next steps, you can specify who should be the recipient of these automatic notifications. Here you can enter specific users or user groups such as doctors. As a contact medium, you can select notification in Climedo and/or by email. In the final step, you can preview what the notification will look like or you have the option of customizing it again (see figure 2).
Fig 2. Preview of notification that selected users or user groups will receive with the corresponding notification information
When a serious adverse event is created in the study, the selected individuals or user groups receive a notification in Climedo and/or by email accordingly. This way, they know that an SAE has been created and they can easily view the new SAE in Climedo via a link in the message.
Form-specific events
As for general events, you can also create notifications that relate to a specific input form. This allows you to be notified when an input form has been completed, changed or made visible. One special feature is that you can also create notifications when a form has not been completed within a certain period of time after becoming available or being updated.
Field-specific events
You can even go one level deeper and create individual notifications for input fields. Here you have a whole range of options such as “is empty”, “is not equal to” and many more. You can also compare input fields with each other.
Additional Features: Slider input field (Visual Analogue Scale (VAS)), Hierarchical signatures, Display of assigned users, Select input fields for reuse
With the current update, not only individual notifications were added, but also other important features that improve the user experience:
For example, using the Visual Analogue Scale (VAS), it is now possible for study participants to show their subjective opinions and assessments with a slider, which you can customize with various implementation options.
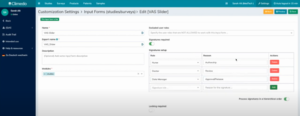
With the new hierarchical signatures feature, you can ensure that all required signatures are captured in the correct order. This helps to make the review process of your study data as efficient as possible. You can define exactly who should carry out and sign which steps in which order (see Figure 3).
Fig. 3. Setting up hierarchical signatures in Climedo
It’s already possible to assign patients to specific users in Climedo to organize your work within the team. Now, you can also view assigned users in the overview and quickly adjust responsibilities in the team. Pro tip: Use the individualized notifications to make things even more efficient!
In order to reduce unnecessary work, you can now decide which input fields you want to save for later reuse and which you don’t. This keeps your workspace organized and you only keep the input fields that you really want to reuse.
Try out new features in Climedo right away!
Can’t imagine the new features in detail yet? Don’t worry! Simply arrange a product demo, secure your free trial or get in touch with your Customer Success contact! We look forward to hearing from you.