Climedo Keeps Getting Better: New Product Features Q2-2021

DATE
June 08, 2021
AUTHOR
Tereza | Senior Customer Success Manager
Already using Climedo or simply interested in what our software has to offer? Read on, because in the second quarter of 2021, we’ve added many new and exciting product features which will help you to manage your projects even better!
What’s it about? – Quick overview:
- Complete renewal of our query management
- Further development of our ePRO module
- Additional features: Reference date, input management
Complete renewal of our query management
Excellent data quality and compliance with the principles of Good Clinical Practice (GCP) is key for your data capture projects. Electronic data capture (EDC) frees you of transmission errors or missing values that significantly impair data quality and subsequent analyses. But what if you do notice an error in your data or simply have a query about an entry that was made? Effective query management is necessary to quickly and easily correct data errors in your EDC software or to query missing values. Therefore, the focus in Q2 was mainly on the improvement of our query management and we are now very pleased to present several new, helpful features!
Clear overviews
In practice, it can be difficult to keep track of all queries. Therefore, Climedo now offers several options to manage your queries. This way, you can easily view your queries in the view that is most helpful for your goals. Keep an overview of all current events and react in a targeted way, and only where necessary. To ensure the highest data quality in detail, it is possible to create one query per data point. In addition, you can track the information history directly at the data point and mark queries as ‘done today’ – and the participant automatically disappears from your to-do list.
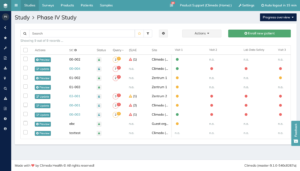
In the study overview, you’ll find the number of all queries per study subject. As you can assess the processing status per participant on the basis of the colour category, this view is ideally suited to the question of what the query status looks like in the overall study or how far it has progressed for individual participants. If you would like an overview of all queries from one or even several studies, it is best to use the monitoring section (see Figure 1). Thanks to the filter function, you can also work specifically here and, for example, display all queries ‘in progress’ per study and center.
Fig 1. Monitoring section in Climedo in an exemplary phase IV trial.
Chat function in the history
To ensure that no errors occur in the query cycle, you can follow it step by step, chronologically and with a comment function, from the creation of a query to its closure. This means that each step can be traced by recording relevant data such as user, time, status and comment.
Status
Our color categories have already been briefly mentioned above – in fact, different codes and colour categories help you to visually sort your queries. There are three status categories based on the traffic light system:
Red: open
Yellow: in progress
Green: closed
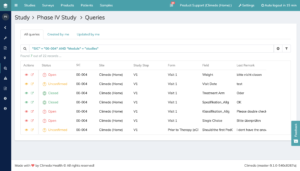
For a granular query cycle, there are even five statuses (see Figure 2):
Open: The user (monitor) sets a query with a comment.
Unconfirmed: The user disagrees with the comment.
Confirmed: The user agrees with the comment and will process the query.
Resolved: The user has made the required changes and indicates that the query has been resolved. The query itself has not yet been finally closed.
Closed: The monitor confirms the request as solved and closes the query.
Fig. 2. Status overview in the patient view showing all queries for the selected patient.
Further development of our ePRO module
For our ePRO module, there have also been many improvements. For example, users can now enjoy an updated layout of the email template. Just one click on the ePRO button takes subjects straight to the corresponding web link for data entry.
Long-term studies are very valuable for long-term risk assessment and these are now facilitated by the extension of the planning horizon: By increasing it to 5,000 days, you can send ePROs up to 14 years into the future and thus also monitor patient safety on a long-term basis as well as record data on participations in the time frame of years-long studies.
In terms of accessibility and user-friendliness, there have been a number of changes: Participants now have the option to adjust the font size to their individual needs using a slider. If information is missing after filling out the form, the participant is automatically guided to the missing information after saving – one after the other, so that they can easily complete it.
If you’d like to give your participants the opportunity to send you individual enquiries, you can now set up any ‘Reply-to’ email address when entering the ePRO steps in a study. All enquiries from your participants will then be forwarded to this address and you can, for example, address any concerns about participating in the study. Of course, this function is optional, so you can decide for yourself whether you want to provide participants with an email address for enquiries.
Additional features: Reference date, input management
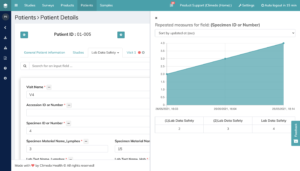
In addition, you can now add a reference date to an input form in the patient module in order to chronologically consolidate identical input forms based on this date in case of multiple use. This feature allows you to identify trends in laboratory parameters by displaying laboratory parameters as repetitive values in chronological order as a graph (see Figure 3).
Fig 3. Visualisation of repetitive values as a graph to see the trend in the data.
After data entry, the already mentioned function of setting participants to ‘mark as done today’ is also available to you in the input management of the eCRF module. This allows you to see the current status of your data processing at any time and you can easily focus on the pending data processing by filtering out processed data. And the best thing is: Climedo works with you and unmarks all data at midnight, so that you can start a new edit the next day.
Try out new features directly in Climedo!
Can’t imagine the new features in detail yet? Don’t worry! Simply arrange a product demo, secure your free trial or get in touch with your Customer Success contact! We look forward to hearing from you.