How PMCF Can Create Value for Medical Device Companies
DATE
May 04, 2020
AUTHOR
Benjamin Sauer | VP Engineering
Post-market Clinical Follow-up (PMCF) is a relatively new requirement in the European MedTech sector. Until recently, it had only been mandatory for higher risk classes (as based on MEDDEV 2.12-2), but under the European Medical Device Regulation (EU MDR), it has become compulsory for all device risk classes. PMCF is defined as a continuous process that “identifies and investigates residual risks associated with medical devices that are on the market” and “updates the clinical evaluation in a life-cycle approach”.
Before the EU MDR came into force, PMCF activities were typically the responsibility of Quality Management and/or Regulatory Affairs departments undertaken by medical device manufacturers of the highest risk classes. Now, however, PMCF data collection needs to become a proactive process which is often carried out by several departments. For example, Sales and Marketing departments collect and utilize data for improving product messaging (e.g. through literature reviews, online forums, or social media), and user feedback can be mined for new Business Development insights.
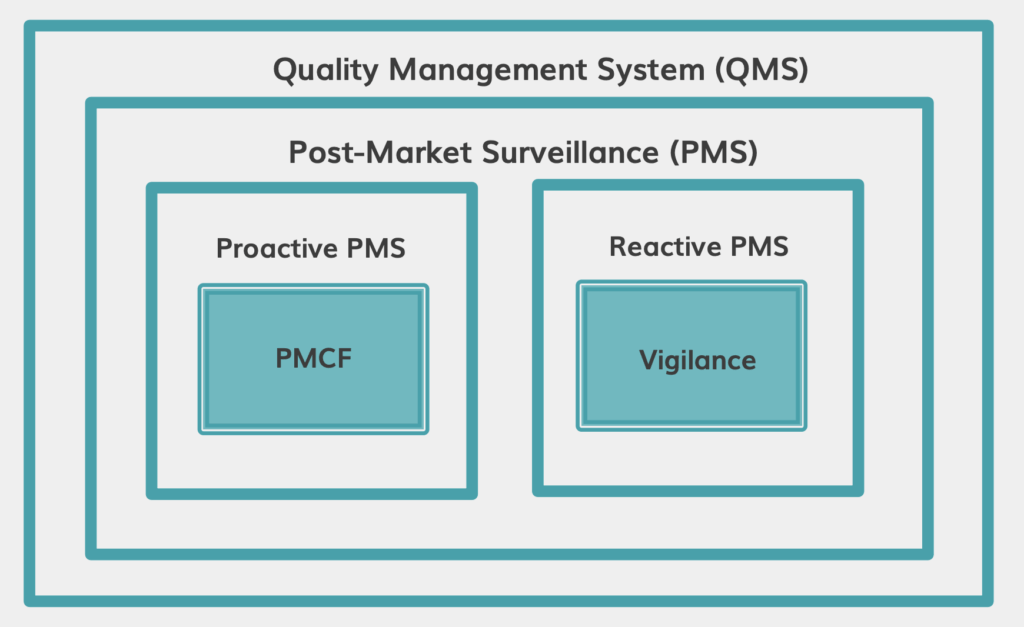
PMS and PMCF as part of QMS
Although PMCF requirements under EU MDR law will undoubtedly increase costs in the short-term, companies can leverage these efforts as positive investments in the long-term. Below, we have compiled four ways in which PMCF can create value for MedTech companies.
Benefit 1: Continuous Real-World Evidence (RWE) from End Users
Leveraging clinical data from a wide variety of sources is imperative for robust PMCF. For devices of lower classes, an entire PMCF study may not be required, but information from various sources can be utilized to adequately portray a device’s risk-benefit ratio. A thoroughly formulated PMCF can put manufacturers in direct contact with both physicians and patients that use the device. Patients can be encouraged to give performance and safety feedback to manufacturers when given the chance to do so in an intuitive manner. In today’s age of digitalization, much of this data can be collected electronically. For example, survey links can be easily sent out via email or SMS and end-user feedback can be collected using ePRO and eCOA. This will allow for ongoing device improvements and ultimately, a stronger competitive advantage in the market.
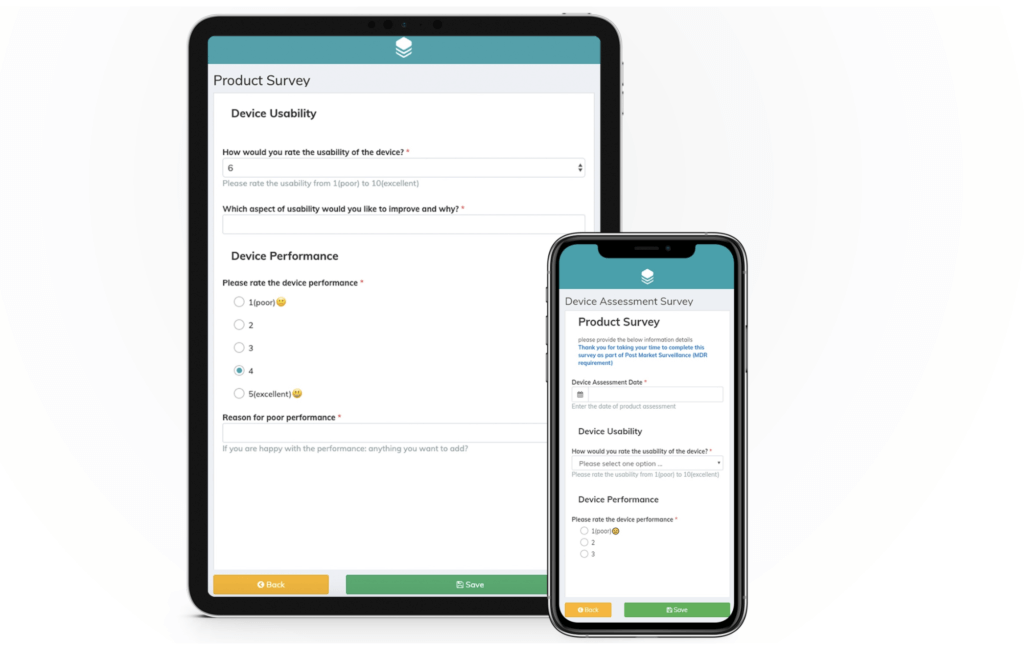
Collecting PMCF data via online user surveys
Benefit 2: New Device Indications (Usages) with PMCF
Once a feedback loop has been established between the manufacturer and end-users, it can be utilised to test and record the medical device’s behavior in different settings as part of PMCF. A great advantage is that possible non-compliant off-label uses can be flagged and reported to the Competent Authority. Often, this can also be a catalyst for discovering potential new indications for the device while complying with regulatory demands. Manufacturers can test these possible uses in the next design change and integrate them into the ‘Instructions for Use’ as a valid, tested and compliant indication.
Benefit 3 : Improved Product Messaging Across Different Departments
Even devices that have undertaken a full-scale clinical study to obtain the CE mark may not be well-received by end users skeptical of a device’s clinical efficacy. Collecting post-market data can help remove this lack of confidence. Common examples include benchmarking the device’s performance against a competitor’s, or presenting evidence-based outcomes at industry trade shows. Moreover, the data can be valuable in optimizing one’s pricing model, thereby improving device turnover. Sales and marketing departments can benefit from the commercial aspects of PMCF by drawing on usability feedback, quality of life (QOL) data, and patient testimonials to improve product messaging and marketing claims. This can help improve acceptance of the product in the market.
Benefit 4: Use PMCF to Build a Network of Trusted Medical Advisors
Clinical staff in hospitals use a staggering array of medical devices on a daily basis and, with the advent of the new EU MDR, will now be expected to receive an overwhelming amount of requests for providing clinical data for PMCF. As healthcare personnel are already stretched on time and resources, relying on ‘cold calls’ to collect PMCF data is not a sustainable strategy. Since gathering clinical data for PMCF must be undertaken throughout the lifecycle of the device, manufacturers can leverage the regulations of the EU MDR to their advantage. Data gathering for PMCF can be used as a basis to build a network of dedicated Key Opinion leaders (KOL’s) and clinical experts who can work with your device and are incentivized to provide clinical data on its performance. Since PMCF activities require robust clinical data, a medical advisory board of KOL’s and clinical experts bring value by providing real-world evidence of the device in the post-market setting.
The EU MDR ushers a new era of regulations that call for the restructuring of existing compliance mechanisms. PMCF is now considered a prerequisite for all device classes and will be integral for maintaining the CE mark and to pass unannounced audits by Notified Bodies (NBs). While costs might increase in the short term, companies would do well to shift their perception of PMCF from an added burden to an extension of their product life cycle management. PMCF activities can bring value by establishing a feedback loop between the manufacturer and the end-user which can be leveraged as advantages in many ways. A well-formulated PMCF shows regulatory authorities that quality is being assessed proactively rather than as an afterthought. PMCF data can support evidence-based outcomes and provide novel insights into real-world use of devices which can be utilized for better marketing and business development. A robust PMCF can create value across organizational processes by updating clinical evaluations and documenting residual risk. By doing so, it can guide regulatory pathways, maximize commercialization opportunities and drive innovation.
The core of a robust and effective PMCF is to gather clinical data efficiently. Manufacturers must therefore be able to easily capture and store clinical data in compliance with all the required standards and regulations. Climedo can streamline your data collection processes for PMCF through a secure, holistic, and connected platform. This way, data becomes accessible for internal purposes, and can be leveraged for product messaging and business development next to providing information for audit purposes.
Want to find out more? Get access to your personal trial account – free and with no strings attached!
Read more by Marius on Medium.






