Leveraging Diversity to Make a Difference in Clinical Trials

DATE
December 15, 2021
AUTHOR
Benjamin Sauer | VP Engineering
Diversity and inclusion are a big part of almost every company’s culture these days. The aim is to actively ensure that opportunities are equally distributed and that no one is put at a disadvantage in accessing career opportunities due to factors such as gender, age or ethnicity. In the clinical environment, however, there is still one area that needs urgent action in terms of diversity: Clinical trials.
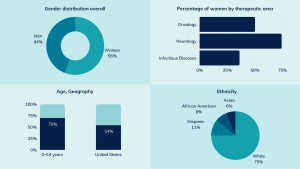
In 2020, the Food and Drug Admission (FDA) approved 53 new drugs. According to the 2020 Summary Report from the Drug Trials Snapshot series published by the FDA, 75% of the patients in the admission trials for these drugs were white. The African American group accounted for 8%, 6% of patients had an Asian ethnicity, and 11% were of Hispanic heritage. In terms of age, only 30% of patients were 65 years of age or older, yet it is the older population that tends to suffer from conditions which require treatment. While the gender distribution was generally relatively balanced with 56% women, a closer look at specific diseases reveals an imbalance: For drugs used to treat neurological diseases, 74% of patients were female, whereas for infectious diseases, only 36% were female (Fig. 1).
Figure 1: Sample composition in registration trials of drugs approved by the FDA in 2020. Source: FDA Drug Trials Snapshots Report (2020).
Which criteria play a role?
In order to assess the degree of diversity in a clinical trial, various characteristics of the study sample need to be considered. Age, gender and ethnicity come immediately to mind and these are important demographic criteria, as is the place of residence of the patients. However, there are also non-demographic criteria that need to be considered, such as comorbidities, disabilities, patients with organ dysfunctions, individuals with extremely low or high body mass index (BMI), and populations with low-prevalence conditions.
Why do we need diversity in clinical trials?
The representativeness of the sample plays an essential role in clinical trials. It allows the results of a small sample of people to be extrapolated to a larger group of people. Since it is not possible for every person in the world to participate in every study (given the large number of clinical trials being conducted on a wide variety of topics), this representativeness is the be-all and end-all for successful studies. And this is precisely why diversity is important, as homogeneous subject groups can lead to biased research results and thus to drugs and medical products that are less effective or even unsuitable for some patient groups.
Evidence for numerous medical devices support this – where differences in efficacy related to ethnicity have been found. For example, differences in skin structure and physiology can have an impact, particularly on dermatological products.
Equally important for the evaluation of the efficacy of medical devices and drugs is the consideration of age. Here, too, some results indicate different efficacy across different age groups. Factors such as altered bone density, changes in metabolism and digestion must be taken into account here.
The last example in this small selection of existing research data is rare diseases. Since only a small, geographically often scattered group of patients is affected by these diseases, the effort must be maximized in order to attract as many patients as possible to participate in a clinical trial.
How do you improve diversity in clinical trials?
The first thing worth doing is to broaden the eligibility criteria in a trial. Often, the inclusion criteria are very narrow because it’s necessary to protect patients from risks of participation that clearly outweigh the benefits of the study. This is particularly the case in pivotal trials for patients with hepatic and renal impairment, as there is little information available on how dosing should be in these particular cases. Pregnant and breastfeeding patients are also often excluded from studies due to inadequate reference values. However, patients are also excluded from trials by default without valid clinical or scientific justification, e.g. due to old age, extreme weight or HIV infection. An expansion of the inclusion criteria can already lead to more diversity in the sample.
On the other hand, some patients are not able to participate in a study because they only have time to participate in the evening or at the weekend due to their working hours, or because they are unable to travel to the study site due to their illness or age. This is where the FDA’s second recommendation comes into play, namely to switch to a different study design in order to improve recruitment and make participation as pleasant as possible for patients.
The German Council of Science has also noticed the poor anchoring of patient centricity in Germany and has therefore recommended that clinical trials should be designed with more patient involvement and that the patient perspective should be consistently taken into account in clinical studies.
Decentralized clinical trials for more patient centricity and diversity
In order to achieve the above-mentioned patient centricity and to make participation in clinical trials as convenient as possible for patients, decentralized clinical trials (DCTs) are an optimal form of study. Through the use of telemedicine, mobile or local healthcare providers and/or mobile technologies, these trials are not tied to a specific geographic location and thus allow a larger and more diverse group to join the trial. There is a wide range of DCTs from hybrid intermediate forms that include only decentralized elements to fully virtual studies. Each decentralized element facilitates participation in the study, often increasing not only locational flexibility but also temporal flexibility.
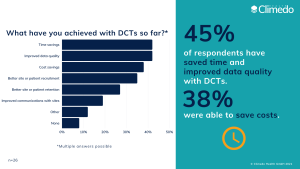
Successful DCTs cannot be conducted without digital solutions. A secure, cloud-based system offers the possibility to monitor the study remotely in real time and to automate study processes. And the implementation of DCTs is also worthwhile for sponsors: time savings, improved data quality and cost savings are the top three benefits that can be achieved with DCTs. However, patients also experience benefits, as a large part of the required study data can be captured from the comfort of their homes. This was also reflected in our recent survey on the status of DCT adoption: better recruitment and retention of patients and sites were among the most important benefits of DCT use (Fig. 2).
Figure 2: Benefits achieved through DCT use.
Conclusion
Diversity helps to make the results of clinical trials transferable to the general population. However, as of today, a lack of diversity in study samples is a major problem that needs to be solved in the coming years. Expanding the patient selection criteria of studies is an important step, as is choosing a study form that makes it as comfortable as possible for patients to participate. Decentralized clinical trials offer an unprecedented level of patient centricity and, because of their decentralized nature, enable even those patients to participate who would normally have difficulty travelling to the study site.