Preliminary Survey Results on the True Cost of the EU MDR

DATE
August 11, 2020
AUTHOR
Benjamin Sauer | VP Engineering
We recently launched our EU MDR survey on the true costs of the new regulation. If you or your customers are affected by the new regulation and have not yet participated, please take a few minutes to do so now! We will share the results with everyone who leaves their email address at the end of the survey.
Having already reached over 80 survey participants, most of whom are medical device manufacturers, we’re pleased to share some of the preliminary results with you. Initially, we will focus on four of the 15 questions:
- How many additional hours per week are companies investing in meeting the EU MDR demands?
- Which activities do companies spend most of their time on?
- What is the total investment in the EU MDR fulfilment for MedTech companies?
- What proportion of PMCF studies is currently automated?
Time is money
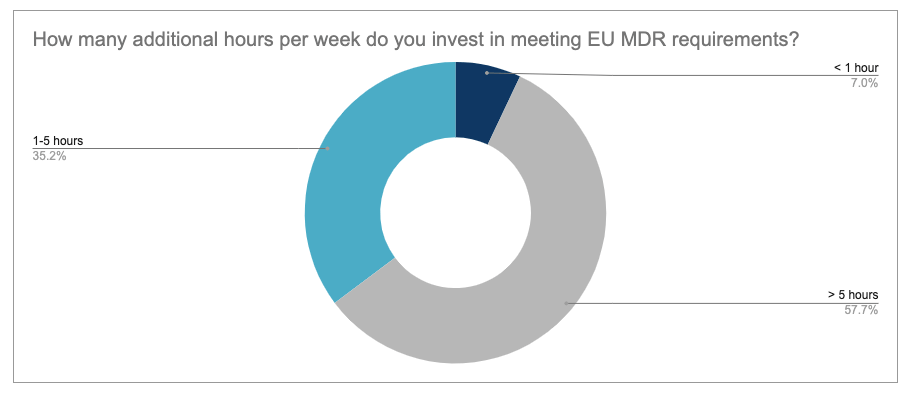
More than half of participants (57%) stated that they invest more than 5 hours per week in complying with the new regulation. More than one third (35%) invest 1 to 5 hours per week and only 7% invest less than 1 hour.
Most time is spent on understanding the requirements
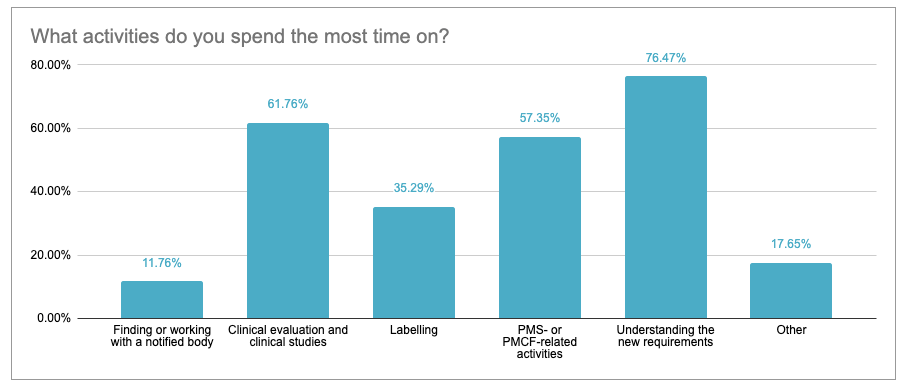
For 76.5% of participants, understanding the new requirements is particularly time consuming. In addition, clinical evaluations or clinical studies (61.7%) and PMS and PMCF activities (57.3%) were mentioned. Labelling and finding a Notified Body were considered to be slightly less time consuming, with 35% and 11.7% respectively. Other areas include gap analyses (MDD-MDR), selecting harmonised standards, the development of a QMS, consulting advice from experts and conformity assessment.
EU MDR still an expensive matter
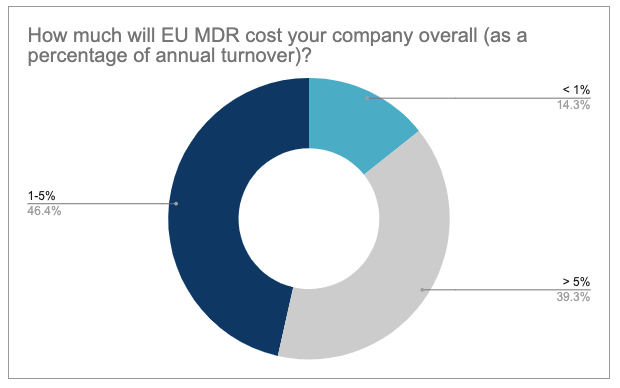
We were also interested to find out what proportion of their annual turnover medical device manufacturers currently have to invest in meeting the requirements. We already asked this question in our first survey back in spring of 2020. This time, 46% believed that they would have to invest 1-5% of their turnover in meeting the requirements and 39% even believed that it would be more than 5%. Just 14% think it will be less than 1%. By contrast, 48% believed they would have to invest 1-5% of their annual turnover, 31.6% believed it would be over 5% and 20.4% thought it would be less than 1%. This means that the participants are now even estimating the overall costs to be higher than just a few months ago.
Room for improvement in terms of PMCF automation
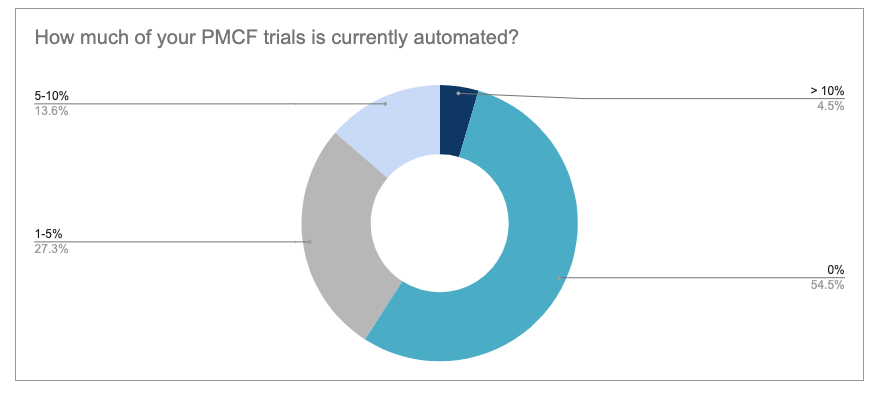
Another area that is particularly close to our hearts here at Climedo is the automation of PMCF studies as part of post-market surveillance. More than half of the current respondents (54.5%) do not yet have any automated processes within their PMCF activities. However, 27.3% have already automated a small part (1-5%), almost a quarter (13.6%) between 5 and 10% and a few (4.5%) even more than 10%. This is encouraging, but there is clearly still room for improvement for the majority of manufacturers. Especially in view of the EU MDR, it will be almost impossible to meet the requirements with analogous processes such as paper or spreadsheets. Automated PMCF processes can also save enormous amounts of time and money.
What’s the situation within your company or for your customers? We would be really grateful if you could take a few minutes to share your opinion in our survey. The results will be sent to you free of charge afterwards.
Survey from spring 2020: Status of MDR implementation
Between February and March of 2020, we conducted an initial survey on the status of EU MDR implementation. The results showed that many MedTech companies were not yet prepared for the new requirements and that postponing the regulation’s start date by 12 months will certainly have been a relief for most. Download the results here for free: